Abstract
The medical use of low level laser (LLL) irradiation has been occurring for decades, primarily in the area of tissue
healing and inflammatory conditions. Despite little mechanistic knowledge, the concept of a non-invasive, nonthermal
intervention that has the potential to modulate regenerative processes is worthy of attention when searching
for novel methods of augmenting stem cell-based therapies. Here we discuss the use of LLL irradiation as a
“photoceutical” for enhancing production of stem cell growth/chemoattractant factors, stimulation of angiogenesis,
and directly augmenting proliferation of stem cells. The combination of LLL together with allogeneic and autologous
stem cells, as well as post-mobilization directing of stem cells will be discussed.
Introduction (Personal Perspective)
We came upon the field of low level laser (LLL) therapy
by accident. One of our advisors read a press release
about a company using this novel technology of specific
light wavelengths to treat stroke. Given the possible role
of stem cells in post-stroke regeneration, we decided to
cautiously investigate. As a background, it should be
said that our scientific team has been focusing on the
area of cord blood banking and manufacturing of disposables
for processing of adipose stem cells for the past 3
years. Our board has been interested in strategically
refocusing the company from services-oriented into a
more research-focused model. An unbiased exploration
into the various degenerative conditions that may be
addressed by our existing know-how led us to explore
the condition of chronic obstructive pulmonary disease
(COPD), an umbrella term covering chronic bronchitis
and emphysema, which is the 4th largest cause of death
in the United States. As a means of increasing our probability
of success in treatment of this condition, the
decision was made to develop an adjuvant therapy that
would augment stem cell activity. The field of LLL therapy
attracted us because it appeared to be relatively
unexplored scientific territory for which large amounts
of clinical experience exist. Unfortunately, it was difficult
to obtain the cohesive “state-of-the-art” description of
the molecular/cellular mechanisms of this therapy in
reviews that we have searched. Therefore we sought in
this mini-review to discuss what we believe to be relevant
to investigators attracted by the concept of “regenerative
photoceuticals”. Before presenting our synthesis
of the field, we will begin by describing our rationale for
approaching COPD with the autologous stem cell based
approaches we are developing.
COPD as an Indication for Stem Cell Therapy
COPD possesses several features making it ideal for
stem cell based interventions: a) the quality of life and
lack of progress demands the ethical exploration of
novel approaches. For example, bone marrow stem cells
have been used in over a thousand cardiac patients with
some indication of efficacy [1,2]. Adipose-based stem
cell therapies have been successfully used in thousands
of race-horses and companion animals without adverse
effects [3], as well as numerous clinical trials are
ongoing and published human data reports no adverse
effects (reviewed in ref [4]). Unfortunately, evaluation of
stem cell therapy in COPD has lagged behind other
areas of regenerative investigation; b) the underlying
cause of COPD appears to be inflammatory and/or
immunologically mediated. The destruction of alveolar
tissue is associated with T cell reactivity [5,6], pathological
pulmonary macrophage activation [7], and auto-antibody
production [8]. Mesenchymal stem cells have been
demonstrated to potently suppress autoreactive T cells
[9,10], inhibit macrophage activation [11], and autoantibody
responses [12]. Additionally, mesenchymal stem
cells can be purified in high concentrations from adipose
stromal vascular tissue together with high concentrations of T regulatory cells [4], which in animal
models are approximately 100 more potent than peripheral
T cells at secreting cytokines therapeutic for COPD
such as IL-10 [13,14]. Additionally, use of adipose
derived cells has yielded promising clinical results in
autoimmune conditions such as multiple sclerosis [4];
and c) Pulmonary stem cells capable of regenerating
damaged parenchymal tissue have been reported [15].
Administration of mesenchymal stem cells into neonatal
oxygen-damaged lungs, which results in COPD-like
alveoli dysplasia, has been demonstrated to yield
improvements in two recent publications [16,17].
Based on the above rationale for stem cell-based
COPD treatments, we began our exploration into this
area by performing several preliminary experiments and
filing patents covering combination uses of stem cells
with various pharmacologically available antiinflammatories,
as well as methods of immune modulation. These
have served as the basis for two of our pipeline candidates,
ENT-111, and ENT-894. As a commerciallyoriented
organization, we needed to develop a therapeutic
candidate that not only has a great potential for efficacy,
but also can be easily implemented as part of the
standard of care. Our search led us to the area of low
level laser (LLL) therapy. From our initial perception as
neophytes to this field, the area of LLL therapy has been
somewhat of a medical mystery. A pubmed search for
“low level laser therapy” yields more than 1700 results,
yet before stumbling across this concept, none of us, or
our advisors, have ever heard of this area of medicine.
On face value, this field appeared to be somewhat of a
panacea: clinical trials claiming efficacy for conditions
ranging from alcoholism [18], to sinusitis [19], to
ischemic heart disease [20]. Further confusing was that
many of the studies used different types of LLL-generating
devices, with different parameters, in different model
systems, making comparison of data almost impossible.
Despite this initial impression, the possibility that a simple,
non-invasive methodology could exist that augments
regenerative potential in a tissue-focused manner
became very enticing to us. Specific uses envisioned, for
which intellectual property was filed included using light
to concentrate stem cells to an area of need, to modulate
effects of stem cells once they are in that specific
area, or even to use light together with other agents to
modulate endogenous stem cells.
The purpose of the current manuscript is to overview
some of the previous work performed in this area that was
of great interest to our ongoing work in regenerative medicine.
We believe that greater integration of the area of
LLL with current advancements in molecular and cellular
biology will accelerate medical progress. Unfortunately, in
our impression to date, this has been a very slow process.
What is Low Level Laser Irradiation?
Lasers (Light amplification by stimulated emission of
radiation) are devices that typically generate electromagnetic
radiation which is relatively uniform in wavelength,
phase, and polarization, originally described by Theodore
Maiman in 1960 in the form of a ruby laser [21]. These
properties have allowed for numerous medical applications
including uses in surgery, activation of photodynamic
agents, and various ablative therapies in cosmetics that are
based on heat/tissue destruction generated by the laser
beam [22-24]. These applications of lasers are considered
“high energy” because of their intensity, which ranges
from about 10-100 Watts. The subject of the current
paper will be another type of laser approach called low
level lasers (LLL) that elicits effects through non-thermal
means. This area of investigation started with the work of
Mester et al who in 1967 reported non-thermal effects of
lasers on mouse hair growth [25]. In a subsequent study
[26], the same group reported acceleration of wound healing
and improvement in regenerative ability of muscle
fibers post wounding using a 1 J/cm2 ruby laser. Since
those early days, numerous in vitro and in vivo studies
have been reported demonstrating a wide variety of therapeutic
effects involving LLL, a selected sample of which
will be discussed below. In order to narrow our focus of
discussion, it is important to first begin by establishing the
current definition of LLL therapy. According to Posten et
al [27], there are several parameters of importance: a)
Power output of laser being 10-3 to 10-1 Watts; b) Wavelength
in the range of 300-10,600 nm; c) Pulse rate from 0,
meaning continuous to 5000 Hertz (cycles per second); d)
intensity of 10-2-10 W/cm(2) and dose of 0.01 to 100 J/
cm2. Most common methods of administering LLL radiation
include lasers such as ruby (694 nm), Ar (488 and 514
nm), He-Ne (632.8 nm), Krypton (521, 530, 568, and 647
nm), Ga-Al-As (805 or 650 nm), and Ga-As (904 nm).
Perhaps one of the most distinguishing features of LLL
therapy as compared to other photoceutical modalities is
that effects are mediated not through induction of thermal
effects but rather through a process that is still not clearly
defined called “photobiostimulation”. It appears that this
effect of LLL is not depend on coherence, and therefore
allows for use of non-laser light generating devices such as
inexpensive Light Emitting Diode (LED) technology [28].
To date several mechanisms of biological action have
been proposed, although none are clearly established.
These include augmentation of cellular ATP levels [29],
manipulation of inducible nitric oxide synthase (iNOS)
activity [30,31], suppression of inflammatory cytokines
such as TNF-alpha, IL-1beta, IL-6 and IL-8 [32-36],
upregulation of growth factor production such as PDGF,
IGF-1, NGF and FGF-2 [36-39], alteration of mitochondrial
membrane potential [29,40-42] due to chromophores found in the mitochondrial respiratory
chain [43,44] as reviewed in [45], stimulation of protein
kinase C (PKC) activation [46], manipulation of NF-!B
activation [47], direct bacteriotoxic effect mediated by
induction of reactive oxygen species (ROS) [48], modification
of extracellular matrix components [49], inhibition
of apoptosis [29], stimulation of mast cell
degranulation [50], and upregulation of heat shock proteins
[51]. Unfortunately these effects have been demonstrated
using a variety of LLL devices in noncomparable
models. To add to confusion, dose-dependency
seems to be confined to such a narrow range or
does not seem to exist in that numerous systems therapeutic
effects disappear with increased dose.
In vitro studies of LLL
In areas of potential phenomenology, it is important to
begin by assessing in vitro studies reported in the literature
in which reproducibility can be attained with some
degree of confidence, and mechanistic dissection is simpler
as compared with in vivo systems. In 1983, one of
the first studies to demonstrate in vitro effects of LLL
was published. The investigators used a helium neon
(He-Ne) laser to generate a visible red light at 632.8 nm
for treatment of porcine granulosa cells. The paper
described upregulation of metabolic and hormone-producing
activity of the cells when exposed for 60 seconds
to pulsating low power (2.8 mW) irradiation [52]. The
possibility of modulating biologically-relevant signaling
proteins by LLL was further assessed in a study using an
energy dose of 1.5 J/cm2 in cultured keratinocytes.
Administration of He-Ne laser emitted light resulted in
upregulated gene expression of IL-1 and IL-8 [53]. Production
of various growth factors in vitro suggests the
possibility of enhanced cellular mitogenesis and mobility
as a result of LLL treatment. Using a diode-based
method to generate a similar wavelength to the He-Ne
laser (363 nm), Mvula et al reported in two papers that
irradiation at 5 J/cm2 of adipose derived mesenchymal
stem cells resulted in enhanced proliferation, viability
and expression of the adhesion molecule beta-1 integrin
as compared to control [54,55]. In agreement with possible
regenerative activity based on activation of stem
cells, other studies have used an in vitro injury model to
examine possible therapeutic effects. Migration of fibroblasts
was demonstrated to be enhanced in a “wound
assay” in which cell monolayers are scraped with a pipette
tip and amount of time needed to restore the
monolayer is used as an indicator of “healing”. The cells
exposed to 5 J/cm2 generated by an He-Ne laser
migrated rapidly across the wound margin indicating a
stimulatory or positive influence of phototherapy.
Higher doses (10 and 16 J/cm2) caused a decrease in
cell viability and proliferation with a significant amount
of damage to the cell membrane and DNA [56]. In
order to examine whether LLL may positively affect
healing under non-optimal conditions that mimic clinical
situations treatment of fibroblasts from diabetic animals
was performed. It was demonstrated that with the
He-Ne laser dosage of 5 J/cm2 fibroblasts exhibited an
enhanced migration activity, however at 16 J/cm2 activity
was negated and cellular damage observed [57]. Thus
from these studies it appears that energy doses from 1.5
J/cm2 to 5 J/cm2 are capable of eliciting “biostimulatory
effects” in vitro in the He-Ne-based laser for adherent
cells that may be useful in regeneration such as fibroblasts
and mesenchymal stem cells.
Studies have also been performed in vitro on immunological
cells. High intensity He-Ne irradiation at 28
and 112 J/cm2 of human peripheral blood mononuclear
cells, a heterogeneous population of T cells, B cells, NK
cells, and monocytes has been described to induce chromatin
relaxation and to augment proliferative response
to the T cell mitogen phytohemaglutin [58]. In human
peripheral blood mononuclear cells (PBMC), another
group reported in two papers that interleukin-1 alpha
(IL-1 alpha), tumor necrosis factor-alpha (TNF-alpha),
interleukin-2 (IL-2), and interferon-gamma (IFNgamma)
at a protein and gene level in PBMC was
increased after He-Ne irradiation at 18.9 J/cm2 and
decreased with 37.8 J/cm2 [59,60]. Stimulation of human
PBMC proliferation and murine splenic lymphocytes
was also reported with He-Ne LLL [61,62]. In terms of
innate immune cells, enhanced phagocytic activity of
murine macrophages have been reported with energy
densities ranging from 100 to 600 J/cm2, with an optimal
dose of 200 J/cm2 [63]. Furthermore, LLL has been
demonstrated to augment human monocyte killing
mycobacterial cells at similar densities, providing a functional
correlation [64].
Thus from the selected in vitro studies discussed, it
appears that modulation of proliferation and soluble factor
production by LLL can be reliably reproduced. However
the data may be to some extent contradictory. For
example, the over-arching clinical rationale for use of
LLL in conditions such as sinusitis [65], arthritis [66,67],
or wound healing [68] is that treatment is associated
with anti-inflammatory effects. However the in vitro studies
described above suggested LLL stimulates proinflammatory
agents such as TNF-alpha or IL-1 [59,60].
This suggests the in vivo effects of LLL may be very
complex, which to some extent should not be surprising.
Factors affecting LLL in vivo actions would include
degree of energy penetration through the tissue, the various
absorption ability of cells in the various tissues, and
complex chemical changes that maybe occurring in
paracrine/autocrine manner. Perhaps an analogy to the
possible discrepancy between LLL effects in vitro versus in vivo may be made with the medical practice of extracorporeal
ozonation of blood. This practice is similar to
LLL therapy given that it is used in treatment of conditions
such as atherosclerosis, non-healing ulcers, and
various degenerative conditions, despite no clear
mechanistic understanding [69-71]. In vitro studies have
demonstrated that ozone is a potent oxidant and inducer
of cell apoptosis and inflammatory signaling [72-74].
In contrast, in vivo systemic changes subsequent to
administration of ozone or ozonized blood in animal
models and patients are quite the opposite. Numerous
investigators have published enhanced anti-oxidant
enzyme activity such as elevations in Mg-SOD and glutathione-
peroxidase levels, as well as diminishment of
inflammation-associated pathology [75-78]. Regardless
of the complexity of in vivo situations, the fact that
reproducible, in vitro experiments, demonstrate a biological
effect provided support for us that there is some
basis for LLL and it is not strictly an area of
phenomenology.
Animal Studies with LLL
As early as 1983, Surinchak et al reported in a rat skin
incision healing model that wounds exposed He-Ne
radiation of fluency 2.2 J/cm2 for 3 min twice daily for
14 days demonstrated a 55% increase in breaking
strength over control rats. Interestingly, higher doses
yielded poorer healing [79]. This application of laser
light was performed directly on shaved skin. In a contradictory
experiment, it was reported that rats irradiated
for 12 days with four levels of laser light (0.0, 0.47, 0.93,
and 1.73 J/cm2) a possible strengthening of wounds tension
was observed at the highest levels of irradiation
(1.73 J/cm2), however it did not reach significance when
analyzed by resampling statistics [80]. In another
wound-healing study Ghamsari et al reported accelerated
healing in the cranial surface of teats in dairy cows
by administration of He-Ne irradiation at 3.64 J/cm2
dose of low-level laser, using a helium-neon system with
an output of 8.5 mW, continuous wave [81]. Collagen
fibers in LLL groups were denser, thicker, better
arranged and more continuous with existing collagen
fibers than those in non-LLL groups. The mean tensile
strength was significantly greater in LLL groups than in
non-LLL groups [82]. In the random skin flap model,
the use of He-Ne laser irradiation with 3 J/cm2 energy
density immediately after the surgery and for the four
subsequent days was evaluated in 4 experimental
groups: Group 1 (control) sham irradiation with He-Ne
laser; Group 2 irradiation by punctual contact technique
on the skin flap surface; Group 3 laser irradiation surrounding
the skin flap; and Group 4 laser irradiation
both on the skin flap surface and around it. The percentage
of necrotic area of the four groups was determined
on day 7-post injury. The control group had an average
necrotic area of 48.86%; the group irradiated on the skin
flap surface alone had 38.67%; the group irradiated
around the skin flap had 35.34%; and the group irradiated
one the skin flap surface and around it had
22.61%. All experimental groups reached statistically significant
values when compared to control [83]. Quite
striking results were obtained in an alloxan-induced diabetes
wound healing model in which a circular 4 cm2
excisional wound was created on the dorsum of the diabetic
rats. Treatment with He-Ne irradiation at 4.8 J/
cm2 was performed 5 days a week until the wound
healed completely and compared to sham irradiated animals.
The laser-treated group healed on average by the
18th day whereas, the control group healed on average
by the 59th day [84].
In addition to mechanically-induced wounds, beneficial
effects of LLL have been obtained in burn-wounds
in which deep second-degree burn wounds were
induced in rats and the effects of daily He-Ne irradiation
at 1.2 and 2.4 J/cm2 were assessed in comparison to
0.2% nitrofurazone cream. The number of macrophages
at day 16, and the depth of new epidermis at day 30,
was significantly less in the laser treated groups in comparison
with control and nitrofurazone treated groups.
Additionally, infections with S. epidermidis and S. aureus
were significantly reduced [85].
While numerous studies have examined dermatological
applications of LLL, which may conceptually be
easier to perform due to ability to topically apply light,
extensive investigation has also been made in the area
of orthopedic applications. Healing acceleration has
been observed in regeneration of the rat mid-cortical
diaphysis of the tibiae, which is a model of post-injury
bone healing. A small hole was surgically made with a
dentistry burr in the tibia and the injured area and LLL
was administered over a 7 or 14 day course transcutaneously
starting 24 h from surgery. Incident energy density
dosages of 31.5 and 94.5 J/cm2 were applied during
the period of the tibia wound healing. Increased angiogenesis
was observed after 7 days irradiation at an
energy density of 94.5 J/cm2, but significantly decreased
the number of vessels in the 14-day irradiated tibiae,
independent of the dosage [86]. In an osteoarthritis
model treatment with He-Ne resulted in augmentation
of heat shock proteins and pathohistological improvement
of arthritic cartilage [87]. The possibility that a
type of preconditioning response is occurring, which
would involve induction of genes such as hemoxygenase-
1 [88], remains to be investigated. Effects of LLL
therapy on articular cartilage were confirmed by another
group. The experiment consisted of 42 young Wistar
rats whose hind limbs were operated on in order to
immobilize the knee joint. One week after operation they were assigned to three groups; irradiance 3.9 W/
cm2, 5.8 W/cm2, and sham treatment. After 6 times of
treatment for another 2 weeks significantpreservation of
articular cartilage stiffness with 3.9 and 5.8 W/cm2 therapy
was observed [89].
Muscle regeneration by LLL was demonstrated in a rat
model of disuse atrophy in which eight-week-old rats
were subjected to hindlimb suspension for 2 weeks,
after which they were released and recovered. During
the recovery period, rats underwent daily LLL irradiation
(Ga-Al-As laser; 830 nm; 60 mW; total, 180 s) to
the right gastrocnemius muscle through the skin. After
2-weeks the number of capillaries and fibroblast growth
factor levels exhibited significant elevation relative to
those of the LLL-untreated muscles. LLL treatment
induced proliferation in satellite cells as detected by
BRdU [90].
Other animal studies of LLL have demonstrated
effects in areas that appear unrelated such as suppression
of snake venom induced muscle death [91],
decreasing histamine-induced vasospasms [92], inhibition
of post-injury restenosis [93], and immune stimulation
by thymic irradiation [94].
Clinical Studies Using LLL
Growth factor secretion by LLL and its apparent regenerative
activities have stimulated studies in radiationinduced
mucositis. A 30 patient randomized trial of carcinoma
patients treated by radiotherapy alone (65 Gy at
a rate of 2 Gy/fraction, 5 fractions per week) without
prior surgery or concomitant chemotherapy suffering
from radiation-induced mucositis was performed using a
He-Ne 60 mW laser. Grade 3 mucositis occured with a
frequency of 35.2% in controls and at 7.6% of treated
patients. Furthermore, a decrease in “severe pain” (grade
3) was observed in that 23.8% in the control group
experienced this level of pain, as compared to 1.9% in
the treatment group [95]. A subsequent study reported
similar effects [96].
Healing ability of lasers was also observed in a study
of patients with gingival flap incisions. Fifty-eight extraction
patients had one of two gingival flap incisions lased
with a 1.4 mW He-Ne (670 nm) at 0.34 J/cm2. Healing
rates were evaluated clinically and photographically.
Sixty-nine percent of the irradiated incisions healed faster
than the control incisions. No significant difference
in healing was noted when patients were compared by
age, gender, race, and anatomic location of the incision
[97]. Another study evaluating healing effects of LLL in
dental practice examined 48 patients subjected to surgical
removal of their lower third molars. Treated patients
were administered Ga-Al-As diode generated 808 nm at
a dose of 12 J. The study demonstrated that extraoral
LLL is more effective than intraoral LLL, which was
more effective than control for the reduction of postoperative
trismus and swelling after extraction of the
lower third molar [98].
Given the predominance of data supporting fibroblast
proliferative ability and animal wound healing effects of
LLL therapy, a clinical trial was performed on healing of
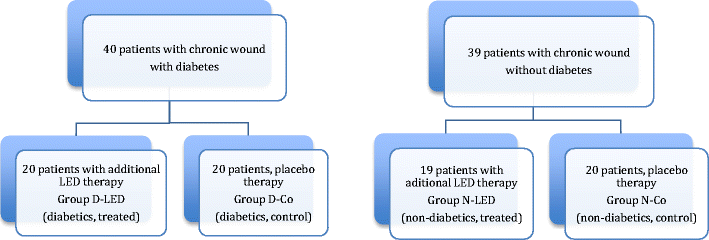
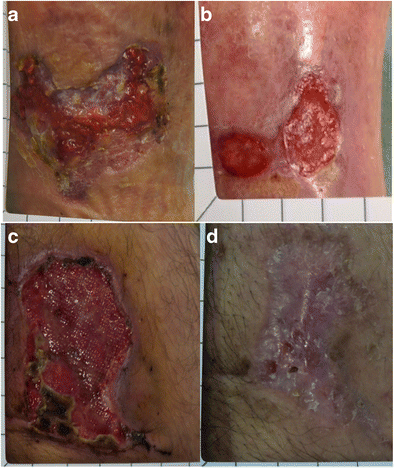
ulcers. In a double-blinded fashion 23 diabetic leg ulcers
from 14 patients were divided into two groups. Phototherapy
was applied (<1.0 J/cm2) twice per week, using a
Dynatron Solaris 705(R) LED device that concurrently
emits 660 and 890 nm energies. At days 15, 30, 45, 60,
75, and 90 mean ulcer granulation and healing rates
were significantly higher for the treatment group as
compared to control. By day 90, 58.3% of the ulcers in
the LLL treated group were fully healed and 75%
achieved 90-100% healing. In the placebo group only
one ulcer healed fully [68].
As previously mentioned, LLL appears to have some
angiogenic activity. One of the major problems in coronary
artery disease is lack of collateralization. In a 39
patient study advanced CAD, two sessions of irradiation
of low-energy laser light on skin in the chest area from
helium-neon B1 lasers. The time of irradiation was 15
minutes while operations were performed 6 days a week
for one month. Reduction in Canadian Cardiology
Society (CCS) score, increased exercise capacity and
time, less frequent angina symptoms during the treadmill
test, longer distance of 6-minute walk test and a
trend towards less frequent 1 mm ST depression lasting
1 min during Holter recordings was noted after therapy
[99].
Perhaps one of the largest clinical trials with LLL was
the NEST trial performed by Photothera. In this double
blind trial 660 stroke patients were recruited and randomized:
331 received LLL and 327 received sham. No
prespecified test achieved significance, but a post hoc
analysis of patients with a baseline National Institutes of
Health Stroke Scale score of <16 showed a favorable
outcome at 90 days on the primary end point (P <
0.044) [100]. Currently Photothera is in the process of
repeating this trial with modified parameters.
Relevance of LLL to COPD
A therapeutic intervention in COPD would require
addressing the issues of inflammation and regeneration.
Although approaches such as administration of bone marrow
stem cells, or fat derived cellular components have
both regenerative and anti-inflammatory activity in animal
models, the need to enhance their potency for clinical
applications can be seen in the recent Osiris’s COPD trial
interim data which reported no significant improvement
in pulmonary function [101]. Accordingly, we sought to
develop a possible rationale for how LLL may be useful as
an adjunct to autologous stem cell therapy.
Table 1 Examples of LLL Properties Relevant to COPD
COPD
Property
LLL Experiment LLL Details Ref
Inflammation In vivo. Decreased joint inflammation in zymosan-induced
arthritis
Semiconductor laser (685 nm and 830 nm) at (2.5 J/cm2)
In vitro. Suppression of LPS-induced bronchial inflammation and
TNF-alpha.
655 nm at of 2.6 J/cm2
In vivo. Carrageenan-induced pleurisy had decreased leukocyte
infiltration and cytokine (TNF-alpha, IL-6, and MCP)
660 nm at 2.1 J/cm2
In vitro. LPS stimulated Raw 264.7 monocytes had reduced gene
expression of MCP-1, IL-1 and IL-6
780 nm diode laser at 2.2 J/cm2)
In vivo. Suppression of LPS-stimulated neutrophil influx,
myeloperoxidase activity and IL-1beta in bronchoalveolar lavage
fluid.
660 nm diode laser at 7.5 J/cm2
In vitro. Inhibition of TNF-alpha induced IL-1, IL-8 and TNF-alpha
mRNA in human synoviocytes
810 nm (5 J/cm2) suppressed IL-1 and TNF, (25 J/cm2) also
suppressed IL-8
In vivo. Reduction of TNF-alpha in diaphragm muscle after
intravenous LPS injection.
4 sessions in 24 h with diode Ga-AsI-Al laser of 650 nm and
a total dose of 5.2 J/cm2
In vivo. Inhibition of LPS induced peritonitis and neutrophil influx 3 J/cm2 and 7.5 J/cm2
Growth Factor Production
In vivo. Upregulation of TGF-b and PDGF in rat gingiva after
incision.
He-Ne laser (632.8 nm) at a dose of 7.5 J/cm2
In vitro. Osteoblast-like cells were isolated from fetal rat calvariae
had increased IGF-1
Ga-Al-As laser (830 nm) at (3.75 J/cm2).
In vitro. Upregulated production of IGF-1 and FGF-2 in human
gingival fibroblasts.
685 nm, for 140 s, 2 J/cm2
Angiogenesis
In vivo. Increased fiber to capillary ratio in rabbits with ligated
femoral arteries.
Gallium-aluminum-arsenide (Ga-Al-As) diode laser, 904 nm
and power of 10 mW
In vitro. Stimulation of HUVEC proliferation by conditioned media
from LLL-treated T cells
820 nm at 1.2 and 3.6 J/cm2.
In vitro. 7-fold increased production of VEGF by cardiomyocytes,
1.6-fold increase by smooth muscle cells (SMC) and fibroblasts.
Supernatant of SMC had increased HUVEC-stimulating potential.
He:Ne continuous wave laser (632 nm). 0.5 J/cm2 for SMC,
2.1 J/cm2 for fibroblasts and 1.05 J/cm2 for cardiomyocytes.
In vitro. Direct stimulation of HUVEC proliferation 670 nm diode device at 2 and 8 J/cm2
Direct Stem Cell Effects
In vivo. LLL precondition significantly enhanced early cell survival
rate by 2-fold, decreased the apoptotic percentage of implanted
BMSCs in infarcted myocardium and increased the number of
newly formed capillaries.
635 nm at 0.96 J/cm2
In vitro. LLL stimulated MSC proliferation, VEGF and NGF
production, and myogenic differentiation after 5-aza induction.
635 nm diode laser at 0.5 J/cm2 for MSC proliferation, 5 J/
cm2 for VEGF and NGF production and for augmentation of
induced myogenic differentiation
In vitro. Increased proliferation of rat MSC. Red light LED 630 nm at 2 and 4 J/cm(2)
In vitro. Augmented proliferation of bone marrow and cardiac
specific stem cells.
GA-Al-As 810 nm at 1 and 3 J/cm2
In vitro/In vivo. Administration of LLL-treated MSC resulted 53%
reduction in infarct size, 5- and 6.3-fold significant increase in cell
density that positively immunoreacted to BrdU and c-kit,
respectively, and 1.4- and 2-fold higher level of angiogenesis and
vascular endothelial growth factor, respectively, when compared
to non-laser-treated implanted cells
Ga-Al-As laser (810 nm wavelength), 1 J/cm2
In vitro. Enhanced proliferation of adipose derived MSC in
presence of EGF.
636 nm diode, 5 J/cm2
Lin et al. Journal of Translational Medicine 2010, 8:16
http://www.translational-medicine.com/content/8/1/16
Table 1: Examples of LLL Properties Relevant to COPD (Continued)
In vitro. Enhanced proliferation and beta-1 integrin expression of
adipose derived MSC.
635 nm diode laser, at 5 J/cm2
Clinical. 660 stroke patients: 331 received LLL and 327 received
sham. No prespecified test achieved significance, but a post hoc
analysis of patients with a baseline National Institutes of Health
Stroke Scale score of <16 showed a favorable outcome at 90
days on the primary end point (P < 0.044).
808 nm. No density disclosed.
Table 1 depicts some of the properties of LLL that provide
a rationale for the combined use with stem cells. One
of the basic properties of LLL seems to be ability to inhibit
inflammation at the level of innate immune activation.
Representative studies showed that LLL was capable of
suppressing inflammatory genes and/or pathology after
administration of lipopolysaccharide (LPS) as a stimulator
of monocytes [102] and bronchial cells [34], in vitro, and
leukocyte infiltration in vivo [103,104]. Inflammation
induced by other stimulators such as zymosan, carrageenan,
and TNF-alpha was also inhibited by LLL
[32,105,106]. Growth factor stimulating activity of LLL
was demonstrated in both in vitro and in vivo experiments
in which augmentation of FGF-2, PDGF and IGF-1 was
observed [36,37,107]. Endogenous production of these
growth factors may be useful in regeneration based on
activation of endogenous pulmonary stem cells [108,109].
Another aspect of LLL activities of relevance is ability to
stimulate angiogenesis. In COPD, the constriction of
blood vessels as a result of poor oxygen uptake is results
in a feedback loop culminating in pulmonary hypertension.
Administration of angiogenic factors has been
demonstrated to be beneficial in several animal models of
pulmonary pathology [110,111]. The ability of LLL to
directly induce proliferation of HUVEC cells [112], as well
as to augment production of angiogenic factors such as
VEGF [113], supports the possibility of creation of an
environment hospitable to neoangiogenesis which is optimal
for stem cell growth. In fact, a study demonstrated in
vivo induction of neocapillary formation subsequent to
LLL administration in a hindlimb ischemia model [114].
The critical importance of angiogenesis in stem cell
mediated regeneration has previously been demonstrated
in the stroke model, where the major therapeutic activity
of exogenous stem cells has been attributed to angiogenic
as opposed to transdifferentiation effects [115].
Direct evidence of LLL stimulating stem cells has been
obtained using mesenchymal stem cells derived both
from the bone marrow and from the adipose tissue
[116,117]. Interestingly in vivo administration of LLL stimulated
MSC has resulted in 50% decrease in cardiac
infarct size [118]. Clinical translation of LLL has been
performed in the area of stroke, in which a 660 patient
trial demonstrated statistically significant effects in post
trial subset analysis [100].
Conclusions
Despite clinical use of LLL for decades, the field is still
in its infancy. As is obvious from the wide variety of
LLL sources, frequencies, and intensities used, no standard
protocols exist. The ability of LLL to induce
growth factor production, inhibition of inflammation,
stimulation of angiogenesis, and direct effects on stem
cells suggests the urgent need for combining this modality
with regenerative medicine, giving birth to the new
field of “regenerative photoceuticals”. Development of a
regenerative treatment for COPD as well as for other
degenerative diseases would be of considerable benefit.
Regarding COPD, such treatment would be life-saving/
life extending for thousands of affected individuals.
Ceasing smoking or not starting to smoke would considerably
impact this disease.
Acknowledgements
The authors thank Victoria Dardov and Matthew Gandjian for critical
discussions and input.
Author details
1Entest BioMedical, San Diego, CA, USA. 2Georgetown Dermatology,
Washington DC, USA. 3Cromos Pharma Services, Longview, WA, USA. 4Center
for the Study of Natural Oncology, Del Mar, CA, USA. 5Department of
Hematology and Medical Oncology, St Francis Hospital and Medical Center,
Hartford, CT, USA. 6Moores Cancer Center, University of California San Diego,
CA, USA. 7Department of Cardiothoracic Surgery, University of Utah, Salt
Lake City, UT, USA.
Authors’ contributions
FL, SFJ, DTA, FR, VB, VG, CAD, RDNC, ANP, EC, DRK contributed to literature
review, analysis and discussion, synthesis of concepts, writing of the
manuscript and proof-reading of the final draft.
Competing interests
David R Koos is a shareholder, as well as Chairman and CEO of Entest Bio.
Feng Lin is research director of Entest Bio. All other authors declare no
competing interest.
Received: 7 January 2010
Accepted: 16 February 2010 Published: 16 February 2010
References
1. Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-
Surma EK, Al-Mallah M, Dawn B: Adult bone marrow-derived cells for
cardiac repair: a systematic review and meta-analysis. Arch Intern Med
2007, 167:989-997.
2. Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM:
Autologous bone marrow stem cells to treat acute myocardial infarction:
a systematic review. Eur Heart J 2008, 29:1807-1818.
3. Vet-Stem Regenerative Veterinary Medicine. http://www.vet-stem.com.
4. Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F, Alfaro M,
Rodriguez JP, Harman RJ, Patel AN, Murphy MP, Lee RR, Minev B: Nonexpanded
adipose stromal vascular fraction cell therapy for multiple
sclerosis. J Transl Med 2009, 7:29.
5. Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R,
Borchers MT: Persistence of lung CD8 T cell oligoclonal expansions upon
smoking cessation in a mouse model of cigarette smoke-induced
emphysema. J Immunol 2008, 181:8036-8043.
6. Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD:
CD8+ T Cells are required for inflammation and destruction in cigarette
smoke-induced emphysema in mice. J Immunol 2007, 178:8090-8096.
7. Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM,
Paquet AC, Erle DJ: A distinctive alveolar macrophage activation state
induced by cigarette smoking. Am J Respir Crit Care Med 2005,
172:1383-1392.
8. Stefanska AM, Walsh PT: Chronic obstructive pulmonary disease: evidence
for an autoimmune component. Cell Mol Immunol 2009, 6:81-86.
9. Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P,
Rico L, Buscher D, Delgado M: Human adipose-derived mesenchymal
stem cells reduce inflammatory and T cell responses and induce
regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis
69:241-248.
10. Lepelletier Y, Lecourt S, Arnulf B, Vanneaux V, Fermand JP, Menasche P,
Domet T, Marolleau JP, Hermine O, Larghero J: Galectin-1 and
Semaphorin-3A are two soluble factors conferring T cell
immunosuppression to bone marrow mesenchymal stem cell. Stem Cells
Dev 2009.
11. Tsyb AF, Petrov VN, Konoplyannikov AG, Saypina EV, Lepechina LA,
Kalsina S, Semenkova IV, Agaeva EV: In vitro inhibitory effect of
mesenchymal stem cells on zymosan-induced production of reactive
oxygen species. Bull Exp Biol Med 2008, 146:158-164.
12. Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S:
Mesenchymal stem cell transplantation reverses multiorgan dysfunction
in systemic lupus erythematosus mice and humans. Stem Cells 2009,
27:1421-1432.
13. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J,
Goldfine AB, Benoist C, Shoelson S, Mathis D: Lean, but not obese, fat is
enriched for a unique population of regulatory T cells that affect
metabolic parameters. Nat Med 2009, 15:930-939.
14. Ogawa Y, Duru EA, Ameredes BT: Role of IL-10 in the resolution of airway
inflammation. Curr Mol Med 2008, 8:437-445.
15. Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O:
Intratracheal transplantation of alveolar type II cells reverses bleomycininduced
lung fibrosis. Am J Respir Crit Care Med 2007, 176:1261-1268.
16. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA,
Kourembanas S: Bone marrow stromal cells attenuate lung injury in a
murine model of neonatal chronic lung disease. Am J Respir Crit Care Med
2009, 180:1122-1130.
17. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M,
Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K,
Abley D, Korbutt G, Archer SL, Thébaud B: Airway delivery of
mesenchymal stem cells prevents arrested alveolar growth in neonatal
lung injury in rats. Am J Respir Crit Care Med 2009, 180:1131-1142.
18. Zalewska-Kaszubska J, Obzejta D: Use of low-energy laser as adjunct
treatment of alcohol addiction. Lasers Med Sci 2004, 19:100-104.
19. Nikitin AV, Esaulenko IE, Shatalova OL: [Effectiveness of laser puncture in
elderly patients with bronchial asthma accompanied by chronic
rhinosinusitis]. Adv Gerontol 2008, 21:424-426.
20. Vasil’ev AP, Strel’tsova NN, Iu Senatorov N: [Laser irradiation in the
treatment of ischemic heart disease]. Vopr Kurortol Fizioter Lech Fiz Kult
2001, 10-13.
21. Maiman TH: Stimulated optical radiation in Ruby. Nature 187:493.
22. Roy D: Ablative facial resurfacing. Dermatol Clin 2005, 23:549-559, viii.
23. Brown MC: An evidence-based approach to patient selection for laser
vision correction. J Refract Surg 2009, 25:S661-667.
24. Brancaleon L, Moseley H: Laser and non-laser light sources for
photodynamic therapy. Lasers Med Sci 2002, 17:173-186.
25. Mester ESB, Tota JG: “Effect of laser on hair growth of mice”. Kiserl
Orvostud 1967, 19:628-631.
26. Mester E, Korenyi-Both A, Spiry T, Tisza S: The effect of laser irradiation on
the regeneration of muscle fibers (preliminary report). Z Exp Chir 1975,
8:258-262.
27. Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M: Low-level
laser therapy for wound healing: mechanism and efficacy. Dermatol Surg
2005, 31:334-340.
28. Vladimirov YA, Osipov AN, Klebanov GI: Photobiological principles of
therapeutic applications of laser radiation. Biochemistry (Mosc) 2004,
69:81-90.
29. Hu WP, Wang JJ, Yu CL, Lan CC, Chen GS, Yu HS: Helium-neon laser
irradiation stimulates cell proliferation through photostimulatory effects
in mitochondria. J Invest Dermatol 2007, 127:2048-2057.
30. Moriyama Y, Nguyen J, Akens M, Moriyama EH, Lilge L: In vivo effects of
low level laser therapy on inducible nitric oxide synthase. Lasers Surg
Med 2009, 41:227-231.
31. Samoilova KA, Zhevago NA, Petrishchev NN, Zimin AA: Role of nitric oxide
in the visible light-induced rapid increase of human skin
microcirculation at the local and systemic levels: II. healthy volunteers.
Photomed Laser Surg 2008, 26:443-449.
32. Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE: Low
level light effects on inflammatory cytokine production by rheumatoid
arthritis synoviocytes. Lasers Surg Med 2009, 41:282-290.
33. Shiba H, Tsuda H, Kajiya M, Fujita T, Takeda K, Hino T, Kawaguchi H,
Kurihara H: Neodymium-doped yttrium-aluminium-garnet laser
irradiation abolishes the increase in interleukin-6 levels caused by
peptidoglycan through the p38 mitogen-activated protein kinase
pathway in human pulp cells. J Endod 2009, 35:373-376.
34. de Lima Mafra F, Costa MS, Albertini R, Silva JA Jr, Aimbire F: Low level
laser therapy (LLLT): attenuation of cholinergic hyperreactivity, beta(2)-
adrenergic hyporesponsiveness and TNF-alpha mRNA expression in rat
bronchi segments in E. coli lipopolysaccharide-induced airway
inflammation by a NF-kappaB dependent mechanism. Lasers Surg Med
2009, 41:68-74.
35. Aimbire F, de Oliveira Ligeiro AP, Albertini R, Correa JC, de Campos
Ladeira CB, Lyon JP, Silva JA Jr, Costa MS: Low level laser therapy (LLLT)
decreases pulmonary microvascular leakage, neutrophil influx and IL-
1beta levels in airway and lung from rat subjected to LPS-induced
inflammation. Inflammation 2008, 31:189-197.
36. Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M: Effects of
low-level He-Ne laser irradiation on the gene expression of IL-1beta,
TNF-alpha, IFN-gamma, TGF-beta, bFGF, and PDGF in rat’s gingiva. Lasers
Med Sci 2008, 23:331-335.
37. Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B: Effects of
laser irradiation on the release of basic fibroblast growth factor (bFGF),
insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from
gingival fibroblasts. Lasers Med Sci 2008, 23:211-215.
38. Schwartz F, Brodie C, Appel E, Kazimirsky G, Shainberg A: Effect of helium/
neon laser irradiation on nerve growth factor synthesis and secretion in
skeletal muscle cultures. J Photochem Photobiol B 2002, 66:195-200.
39. Yu W, Naim JO, Lanzafame RJ: The effect of laser irradiation on the
release of bFGF from 3T3 fibroblasts. Photochem Photobiol 1994,
59:167-170.
40. Zungu IL, Hawkins Evans D, Abrahamse H: Mitochondrial responses of
normal and injured human skin fibroblasts following low level laser
irradiation–an in vitro study. Photochem Photobiol 2009, 85:987-996.
41. Wu S, Xing D, Gao X, Chen WR: High fluence low-power laser irradiation
induces mitochondrial permeability transition mediated by reactive
oxygen species. J Cell Physiol 2009, 218:603-611.
42. Lan CC, Wu CS, Chiou MH, Chiang TY, Yu HS: Low-energy helium-neon
laser induces melanocyte proliferation via interaction with type IV
collagen: visible light as a therapeutic option for vitiligo. Br J Dermatol
2009, 161:273-280.
43. Karu T: Photobiology of low-power laser effects. Health Phys 1989,
56:691-704.
44. Tiphlova O, Karu T: Role of primary photoacceptors in low-power laser
effects: action of He-Ne laser radiation on bacteriophage T4-Escherichia
coli interaction. Lasers Surg Med 1989, 9:67-69.
45. Karu TI: Mitochondrial signaling in mammalian cells activated by red and
near-IR radiation. Photochem Photobiol 2008, 84:1091-1099.
Lin et al. Journal of Translational Medicine 2010, 8:16
http://www.translational-medicine.com/content/8/1/16
46. Zhang L, Xing D, Zhu D, Chen Q: Low-power laser irradiation inhibiting
Abeta25-35-induced PC12 cell apoptosis via PKC activation. Cell Physiol
Biochem 2008, 22:215-222.
47. Aimbire F, Santos FV, Albertini R, Castro-Faria-Neto HC, Mittmann J,
Pacheco-Soares C: Low-level laser therapy decreases levels of lung
neutrophils anti-apoptotic factors by a NF-kappaB dependent
mechanism. Int Immunopharmacol 2008, 8:603-605.
48. Lipovsky A, Nitzan Y, Lubart R: A possible mechanism for visible lightinduced
wound healing. Lasers Surg Med 2008, 40:509-514.
49. Ignatieva N, Zakharkina O, Andreeva I, Sobol E, Kamensky V, Lunin V: Effects
of laser irradiation on collagen organization in chemically induced
degenerative annulus fibrosus of lumbar intervertebral disc. Lasers Surg
Med 2008, 40:422-432.
50. Silveira LB, Prates RA, Novelli MD, Marigo HA, Garrocho AA, Amorim JC,
Sousa GR, Pinotti M, Ribeiro MS: Investigation of mast cells in human
gingiva following low-intensity laser irradiation. Photomed Laser Surg
2008, 26:315-321.
51. Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC,
Yum LW: The effects of low level laser irradiation on osteoblastic cells.
Clin Orthod Res 2001, 4:3-14.
52. Gregoraszczuk E, Dobrowolski JW, Galas J: Effect of low intensity laser
beam on steroid dehydrogenase activity and steroid hormone
production in cultured porcine granulosa cells. Folia Histochem Cytochem
(Krakow) 1983, 21:87-92.
53. Yu HS, Chang KL, Yu CL, Chen JW, Chen GS: Low-energy helium-neon
laser irradiation stimulates interleukin-1 alpha and interleukin-8 release
from cultured human keratinocytes. J Invest Dermatol 1996, 107:593-596.
54. Mvula B, Mathope T, Moore T, Abrahamse H: The effect of low level laser
irradiation on adult human adipose derived stem cells. Lasers Med Sci
2008, 23:277-282.
55. Mvula B, Moore TJ, Abrahamse H: Effect of low-level laser irradiation and
epidermal growth factor on adult human adipose-derived stem cells.
Lasers Med Sci 25:33-39.
56. Hawkins DH, Abrahamse H: The role of laser fluence in cell viability,
proliferation, and membrane integrity of wounded human skin
fibroblasts following helium-neon laser irradiation. Lasers Surg Med 2006,
38:74-83.
57. Houreld N, Abrahamse H: In vitro exposure of wounded diabetic
fibroblast cells to a helium-neon laser at 5 and 16 J/cm2. Photomed Laser
Surg 2007, 25:78-84.
58. Smol’yaninova NK, Karu TI, Fedoseeva GE, Zelenin AV: Effects of He-Ne
laser irradiation on chromatin properties and synthesis of nucleic acids
in human peripheral blood lymphocytes. Biomed Sci 1991, 2:121-126.
59. Funk JO, Kruse A, Neustock P, Kirchner H: Helium-neon laser irradiation
induces effects on cytokine production at the protein and the mRNA
level. Exp Dermatol 1993, 2:75-83.
60. Funk JO, Kruse A, Kirchner H: Cytokine production after helium-neon laser
irradiation in cultures of human peripheral blood mononuclear cells. J
Photochem Photobiol B 1992, 16:347-355.
61. Gulsoy M, Ozer GH, Bozkulak O, Tabakoglu HO, Aktas E, Deniz G, Ertan C:
The biological effects of 632.8-nm low energy He-Ne laser on peripheral
blood mononuclear cells in vitro. J Photochem Photobiol B 2006,
82:199-202.
62. Novoselova EG, Cherenkov DA, Glushkova OV, Novoselova TV,
Chudnovskii VM, Iusupov VI, Fesenko EE: [Effect of low-intensity laser
radiation (632.8 nm) on immune cells isolated from mice]. Biofizika 2006,
51:509-518.
63. Dube A, Bansal H, Gupta PK: Modulation of macrophage structure and
function by low level He-Ne laser irradiation. Photochem Photobiol Sci
2003, 2:851-855.
64. Hemvani N, Chitnis DS, Bhagwanani NS: Helium-neon and nitrogen laser
irradiation accelerates the phagocytic activity of human monocytes.
Photomed Laser Surg 2005, 23:571-574.
65. Moustsen PA, Vinter N, Aas-Andersen L, Kragstrup J: [Laser treatment of
sinusitis in general practice assessed by a double-blind controlled
study]. Ugeskr Laeger 1991, 153:2232-2234.
66. Shen X, Zhao L, Ding G, Tan M, Gao J, Wang L, Lao L: Effect of combined
laser acupuncture on knee osteoarthritis: a pilot study. Lasers Med Sci
2009, 24:129-136.
67. Ekim A, Armagan O, Tascioglu F, Oner C, Colak M: Effect of low level laser
therapy in rheumatoid arthritis patients with carpal tunnel syndrome.
Swiss Med Wkly 2007, 137:347-352.
68. Minatel DG, Frade MA, Franca SC, Enwemeka CS: Phototherapy promotes
healing of chronic diabetic leg ulcers that failed to respond to other
therapies. Lasers Surg Med 2009, 41:433-441.
69. Bocci V, Travagli V, Zanardi I: May oxygen-ozone therapy improves
cardiovascular disorders?. Cardiovasc Hematol Disord Drug Targets 2009,
9:78-85.
70. Bocci V, Borrelli E, Travagli V, Zanardi I: The ozone paradox: ozone is a
strong oxidant as well as a medical drug. Med Res Rev 2009, 29:646-682.
71. Re L, Mawsouf MN, Menendez S, Leon OS, Sanchez GM, Hernandez F:
Ozone therapy: clinical and basic evidence of its therapeutic potential.
Arch Med Res 2008, 39:17-26.
72. Damera G, Zhao H, Wang M, Smith M, Kirby C, Jester WF, Lawson JA,
Panettieri RA Jr: Ozone modulates IL-6 secretion in human airway
epithelial and smooth muscle cells. Am J Physiol Lung Cell Mol Physiol
2009, 296:L674-683.
73. Manzer R, Dinarello CA, McConville G, Mason RJ: Ozone exposure of
macrophages induces an alveolar epithelial chemokine response
through IL-1alpha. Am J Respir Cell Mol Biol 2008, 38:318-323.
74. McDonald RJ, Usachencko J: Neutrophils injure bronchial epithelium after
ozone exposure. Inflammation 1999, 23:63-73.
75. Rodriguez ZZ, Guanche D, Alvarez RG, Rosales FH, Alonso Y, Schulz S:
Preconditioning with ozone/oxygen mixture induces reversion of some
indicators of oxidative stress and prevents organic damage in rats with
fecal peritonitis. Inflamm Res 2009.
76. Zamora ZB, Borrego A, Lopez OY, Delgado R, Gonzalez R, Menendez S,
Hernandez F, Schulz S: Effects of ozone oxidative preconditioning on
TNF-alpha release and antioxidant-prooxidant intracellular balance in
mice during endotoxic shock. Mediators Inflamm 2005, 2005:16-22.
77. Borrego A, Zamora ZB, Gonzalez R, Romay C, Menendez S, Hernandez F,
Montero T, Rojas E: Protection by ozone preconditioning is mediated by
the antioxidant system in cisplatin-induced nephrotoxicity in rats.
Mediators Inflamm 2004, 13:13-19.
78. Martinez-Sanchez G, Al-Dalain SM, Menendez S, Re L, Giuliani A, Candelario-
Jalil E, Alvarez H, Fernandez-Montequin JI, Leon OS: Therapeutic efficacy of
ozone in patients with diabetic foot. Eur J Pharmacol 2005, 523:151-161.
79. Surinchak JS, Alago ML, Bellamy RF, Stuck BE, Belkin M: Effects of low-level
energy lasers on the healing of full-thickness skin defects. Lasers Surg
Med 1983, 2:267-274.
80. Broadley C, Broadley KN, Disimone G, Riensch L, Davidson JM: Low-energy
helium-neon laser irradiation and the tensile strength of incisional
wounds in the rat. Wound Repair Regen 1995, 3:512-517.
81. Ghamsari SM, Taguchi K, Abe N, Acorda JA, Yamada H: Histopathological
effect of low-level laser therapy on sutured wounds of the teat in dairy
cattle. Vet Q 1996, 18:17-21.
82. Ghamsari SM, Taguchi K, Abe N, Acorda JA, Sato M, Yamada H: Evaluation
of low level laser therapy on primary healing of experimentally induced
full thickness teat wounds in dairy cattle. Vet Surg 1997, 26:114-120.
83. Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM: Helium-neon laser in
viability of random skin flap in rats. Lasers Surg Med 2005, 37:74-77.
84. Maiya GA, Kumar P, Rao L: Effect of low intensity helium-neon (He-Ne)
laser irradiation on diabetic wound healing dynamics. Photomed Laser
Surg 2005, 23:187-190.
85. Bayat M, Vasheghani MM, Razavi N, Taheri S, Rakhshan M: Effect of lowlevel
laser therapy on the healing of second-degree burns in rats: a
histological and microbiological study. J Photochem Photobiol B 2005,
78:171-177.
86. Garavello I, Baranauskas V, da Cruz-Hofling MA: The effects of low laser
irradiation on angiogenesis in injured rat tibiae. Histol Histopathol 2004,
19:43-48.
87. Lin YS, Huang MH, Chai CY, Yang RC: Effects of helium-neon laser on
levels of stress protein and arthritic histopathology in experimental
osteoarthritis. Am J Phys Med Rehabil 2004, 83:758-765.
88. Jamieson RW, Friend PJ: Organ reperfusion and preservation. Front Biosci
2008, 13:221-235.
89. Akai M, Usuba M, Maeshima T, Shirasaki Y, Yasuoka S: Laser’s effect on
bone and cartilage change induced by joint immobilization: an
experiment with animal model. Lasers Surg Med 1997, 21:480-484.
Lin et al. Journal of Translational Medicine 2010, 8:16
http://www.translational-medicine.com/content/8/1/16
90. Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T: Lowlevel
laser irradiation promotes the recovery of atrophied gastrocnemius
skeletal muscle in rats. Exp Physiol 2009, 94:1005-1015.
91. Doin-Silva R, Baranauskas V, Rodrigues-Simioni L, da Cruz-Hofling MA: The
ability of low level laser therapy to prevent muscle tissue damage
induced by snake venom. Photochem Photobiol 2009, 85:63-69.
92. Gal D, Chokshi SK, Mosseri M, Clarke RH, Isner JM: Percutaneous delivery of
low-level laser energy reverses histamine-induced spasm in
atherosclerotic Yucatan microswine. Circulation 1992, 85:756-768.
93. Kipshidze N, Sahota H, Komorowski R, Nikolaychik V, Keelan MH Jr:
Photoremodeling of arterial wall reduces restenosis after balloon
angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol 1998,
31:1152-1157.
94. Novoselova EG, Glushkova OV, Cherenkov DA, Chudnovsky VM, Fesenko EE:
Effects of low-power laser radiation on mice immunity. Photodermatol
Photoimmunol Photomed 2006, 22:33-38.
95. Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M,
Dejou J, Tardieu C, Benezery K, Nguyen TD, Laudoyer Y, Dassonville O,
Poissonnet G, Vallicioni J, Thyss A, Hamdi M, Chauvel P, Demard F: Lowenergy
He/Ne laser in the prevention of radiation-induced mucositis. A
multicenter phase III randomized study in patients with head and neck
cancer. Support Care Cancer 1999, 7:244-252.
96. Arun Maiya G, Sagar MS, Fernandes D: Effect of low level helium-neon
(He-Ne) laser therapy in the prevention & treatment of radiation
induced mucositis in head & neck cancer patients. Indian J Med Res 2006,
124:399-402.
97. Neiburger EJ: Rapid healing of gingival incisions by the helium-neon
diode laser. J Mass Dent Soc 1999, 48:8-13, 40..
98. Aras MH, Gungormus M: Placebo-controlled randomized clinical trial of
the effect two different low-level laser therapies (LLLT)-intraoral and
extraoral-on trismus and facial swelling following surgical extraction of
the lower third molar. Lasers Med Sci 2009.
99. Zycinski P, Krzeminska-Pakula M, Peszynski-Drews C, Kierus A, Trzos E,
Rechcinski T, Figiel L, Kurpesa M, Plewka M, Chrzanowski L, Drozdz J: Laser
biostimulation in end-stage multivessel coronary artery disease–a
preliminary observational study. Kardiol Pol 2007, 65:13-21, discussion 22-
13..
100. Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T,
Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J,
Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M,
Streeter J, NeuroThera Effectiveness and Safety Trial-2 Investigators:
Effectiveness and safety of transcranial laser therapy for acute ischemic
stroke. Stroke 2009, 40:1359-1364.
101. Osiris Therapeutics Reports Interim Data for COPD Trial. http://www.
medicalnewstoday.com/articles/155267.php.
102. Gavish L, Perez LS, Reissman P, Gertz SD: Irradiation with 780 nm diode
laser attenuates inflammatory cytokines but upregulates nitric oxide in
lipopolysaccharide-stimulated macrophages: implications for the
prevention of aneurysm progression. Lasers Surg Med 2008, 40:371-378.
103. Correa F, Lopes Martins RA, Correa JC, Iversen VV, Joenson J, Bjordal JM:
Low-level laser therapy (GaAs lambda = 904 nm) reduces inflammatory
cell migration in mice with lipopolysaccharide-induced peritonitis.
Photomed Laser Surg 2007, 25:245-249.
104. Aimbire F, Lopes-Martins RA, Castro-Faria-Neto HC, Albertini R,
Chavantes MC, Pacheco MT, Leonardo PS, Iversen VV, Bjordal JM: Low-level
laser therapy can reduce lipopolysaccharide-induced contractile force
dysfunction and TNF-alpha levels in rat diaphragm muscle. Lasers Med
Sci 2006, 21:238-244.
105. de Morais NC, Barbosa AM, Vale ML, Villaverde AB, de Lima CJ, Cogo JC,
Zamuner SR: Anti-Inflammatory Effect of Low-Level Laser and Light-
Emitting Diode in Zymosan-Induced Arthritis. Photomed Laser Surg 2009.
106. Boschi ES, Leite CE, Saciura VC, Caberlon E, Lunardelli A, Bitencourt S,
Melo DA, Oliveira JR: Anti-Inflammatory effects of low-level laser therapy
(660 nm) in the early phase in carrageenan-induced pleurisy in rat.
Lasers Surg Med 2008, 40:500-508.
107. Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y: Lowintensity
laser irradiation stimulates bone nodule formation via insulinlike
growth factor-I expression in rat calvarial cells. Lasers Surg Med 2007,
39:551-559.
108. Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV,
Jacoby DB, Kicic A, Stick SM, Knight DA: Characterization of side
population cells from human airway epithelium. Stem Cells 2008,
26:2576-2585.
109. Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M,
Young KA, Klemm D, Gebb S, Dempsey EC, West J, Majka S: Neonatal lung
side population cells demonstrate endothelial potential and are altered
in response to hyperoxia-induced lung simplification. Am J Physiol Lung
Cell Mol Physiol 2007, 293:L941-951.
110. Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F,
Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL: Vascular
endothelial growth factor gene therapy increases survival, promotes
lung angiogenesis, and prevents alveolar damage in hyperoxia-induced
lung injury: evidence that angiogenesis participates in alveolarization.
Circulation 2005, 112:2477-2486.
111. Thebaud B: Angiogenesis in lung development, injury and repair:
implications for chronic lung disease of prematurity. Neonatology 2007,
91:291-297.
112. Schindl A, Merwald H, Schindl L, Kaun C, Wojta J: Direct stimulatory effect
of low-intensity 670 nm laser irradiation on human endothelial cell
proliferation. Br J Dermatol 2003, 148:334-336.
113. Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R,
Leon M, Moses J: Low-power helium: neon laser irradiation enhances
production of vascular endothelial growth factor and promotes growth
of endothelial cells in vitro. Lasers Surg Med 2001, 28:355-364.
114. Ihsan FR: Low-level laser therapy accelerates collateral circulation and
enhances microcirculation. Photomed Laser Surg 2005, 23:289-294.
115. Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H,
Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T:
Administration of CD34+ cells after stroke enhances neurogenesis via
angiogenesis in a mouse model. J Clin Invest 2004, 114:330-338.
116. Li WT, Leu YC: Effects of low level red-light irradiation on the
proliferation of mesenchymal stem cells derived from rat bone marrow.
Conf Proc IEEE Eng Med Biol Soc 2007, 2007:5830-5833.
117. Tuby H, Maltz L, Oron U: Low-level laser irradiation (LLLI) promotes
proliferation of mesenchymal and cardiac stem cells in culture. Lasers
Surg Med 2007, 39:373-378.
118. Tuby H, Maltz L, Oron U: Implantation of low-level laser irradiated
mesenchymal stem cells into the infarcted rat heart is associated with
reduction in infarct size and enhanced angiogenesis. Photomed Laser
Surg 2009, 27:227-233.
119. Agaiby AD, Ghali LR, Wilson R, Dyson M: Laser modulation of angiogenic
factor production by T-lymphocytes. Lasers Surg Med 2000, 26:357-363.
120. Zhang H, Hou JF, Shen Y, Wang W, Wei YJ, Hu S: Low Level Laser
Irradiation Precondition to Create Friendly Milieu of Infarcted
Myocardium and Enhance Early Survival of Transplanted Bone Marrow
Cells. J Cell Mol Med 2009.
121. Hou JF, Zhang H, Yuan X, Li J, Wei YJ, Hu SS: In vitro effects of low-level
laser irradiation for bone marrow mesenchymal stem cells: proliferation,
growth factors secretion and myogenic differentiation. Lasers Surg Med
2008, 40:726-733.
doi:10.1186/1479-5876-8-16
Cite this article as: Lin et al.: Lasers, stem cells, and COPD. Journal of
Translational Medicine 2010 8:16.
Original Source:
http://www.translational-medicine.com/content/8/1/16
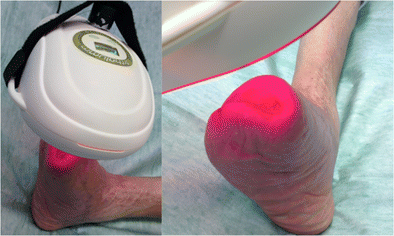
 Glowing red light from High Emissivity Aluminiferous Luminescent Substrate, or HEALS technology has been proven to aid in the healing of human wounds, burns, diabetic skin ulcers and oral mucositis. (NASA/MSFC/Higginbotham)
Glowing red light from High Emissivity Aluminiferous Luminescent Substrate, or HEALS technology has been proven to aid in the healing of human wounds, burns, diabetic skin ulcers and oral mucositis. (NASA/MSFC/Higginbotham)  A nurse in the Bone Marrow Transplant and Cellular Therapy Unit at the University of Alabama at Birmingham Hospital demonstrates use of a WARP 75 device. (NASA/MSFC/Higginbotham)
A nurse in the Bone Marrow Transplant and Cellular Therapy Unit at the University of Alabama at Birmingham Hospital demonstrates use of a WARP 75 device. (NASA/MSFC/Higginbotham)