Start New Query
Treatments for Traumatic Brain Injury With Emphasis on Transcranial Near-Infrared Laser Phototherapy
Larry D Morries, Paolo Cassano, Theodore A Henderson, - This article was published in Neuropsychiatric Disease and Treatment, 20 August 2015 (Publication) 4406
This exceptional research indicated prefered wavelenghts and dosages for treating patients with traumatic brain injuries. The found some surprising results.
View Resource
Abstract:
Traumatic brain injury (TBI) is a growing health concern affecting civilians and military personnel. In this review, treatments for the chronic TBI patient are discussed, including pharmaceuticals, nutraceuticals, cognitive therapy, and hyperbaric oxygen therapy. All available literature suggests a marginal benefit with prolonged treatment courses. An emerging modality of treatment is near-infrared (NIR) light, which has benefit in animal models of stroke, spinal cord injury, optic nerve injury, and TBI, and in human trials for stroke and TBI. The extant literature is confounded by variable degrees of efficacy and a bewildering array of treatment parameters. Some data indicate that diodes emitting low-level NIR energy often have failed to demonstrate therapeutic efficacy, perhaps due to failing to deliver sufficient radiant energy to the necessary depth. As part of this review, we present a retrospective case series using high-power NIR laser phototherapy with a Class IV laser to treat TBI. We demonstrate greater clinical efficacy with higher fluence, in contrast to the bimodal model of efficacy previously proposed. In ten patients with chronic TBI (average time since injury 9.3 years) given ten treatments over the course of 2 months using a high-power NIR laser (13.2 W/0.89 cm2 at 810 nm or 9 W/0.89 cm2 at 810 nm and 980 nm), symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability improved. Symptoms were monitored by depression scales and a novel patient diary system specifically designed for this study. NIR light in the power range of 10-15 W at 810 nm and 980 nm can safely and effectively treat chronic symptoms of TBI. The clinical benefit and effects of infrared phototherapy on mitochondrial function and secondary molecular events are discussed in the context of adequate radiant energy penetration. Keywords: infrared, traumatic brain injury, TBI, transcranial infrared light therapy, transcranial laser therapy
INTRODUCTION
Traumatic brain injury (TBI) has recently moved into the limelight due to the recognition of its impact on professional athletes and military personnel. Yet, TBI is neither a new problem nor limited to those two populations. The Centers for Disease Control and Prevention estimated that 1.5 million Americans sustained TBI annually in 2000.1 As of 2006, the estimates had risen to 1.7 million brain injuries annually.2,3 Undoubtedly, these point prevalence proportions will increase as military personnel return home,4 and the problem of repeated mild TBI (mTBI) becomes more recognized in sports.5 Current estimates of the prevalence of TBI among veterans range from 9.6%6 to 20%,7 with an estimated total of more than 300,000 cases of TBI among military personnel since 2000.4 The current estimates of the combined number of sportsrelated concussions and brain injuries in the US are 1.6-3.8 million annually.8-10 TBI results in a wide spectrum of neurological, psychiatric, cognitive, and emotional consequences. In part, the variation is related to the severity of the injury (mild, moderate, severe TBI), which is stratified based on Glasgow Coma score, periods of unconsciousness, and degrees of amnesia. Furthermore, the diversity of sequalae can be related to the areas of the brain that are injured, the severity of the injury (highly variable within the classification of “mild” and “moderate”), and the evolution of the injury over time due to neuroinflammatory processes.11,12 Additional mechanisms thought to underlie the damage of TBI include decreased mitochondrial function, calcium and magnesium dysregulation, excitotoxicity, disruption of neural networks, free radicalinduced damage, excessive nitric oxide, ischemia, and damage to the blood-brain barrier. Together, these can contribute to a progression of the damage over time. Patients with TBI can experience headache, visual disturbances, dizziness, cognitive impairment, loss of executive skills, memory impairment, fatigue, impulsivity, impaired judgment, emotional outbursts, anxiety, and depression.3,13-23 The situation can be further clouded by secondary and/ or comorbid posttraumatic stress disorder (PTSD), depression, and anxiety,17-25 which can have symptoms that overlap with those described above and appear to be increasingly likely with repetitive concussive or subconcussive brain injury.5,24,26
TREATMENTS FOR TBI
Pharmacological treatments Pharmacological treatment largely targets the neuropsychiatric sequalae of TBI, rather than providing any means of healing or repairing injury. In general, pharmacological treatment is focused on the modulation of major neurotransmitter systems – dopaminergic, serotonergic, noradrenergic, acetylcholinergic, and glutaminergic.20 Disruption of the major neurotransmitter pathways may result from direct injury or excitotoxicity and other cytotoxic mechanisms. The treatment of depression secondary to TBI is often approached with serotonin reuptake inhibitors. Several studies have examined the benefit of sertraline in post- TBI depression.27-29 Other serotonin reuptake inhibitors also have been examined. Tricyclic antidepressants appear to have some use in the treatment of post-TBI depression, although cautious dose titration is required. Patients with TBI are at greater vulnerability to sedation and cholinergic side effects of confusion and memory impairment. With serotonergic agents other than sertraline, cognitive effects also have been reported.30 Similarly, lithium may be a less desirable agent in this population due to sedation and cognitive impairment. Patients with TBI may respond at lower doses and lower blood levels than expected. Modulation of the dopaminergic system may improve alertness, attention, and cognitive processing speed. The stimulants are most commonly used for this purpose. Methylphenidate facilitates the release of dopamine and slows its reuptake. Dextroamphetamine strongly inhibits reuptake of dopamine, slows down the breakdown of dopamine by monoamine oxidase, and somewhat increases the release of dopamine. These subtle differences are sometimes imperceptible to the patient, but at other times, a patient will do best on one or the other stimulant. Increasing dopamine in the reticular activating system leads to enhanced arousal. Increasing dopamine within the frontal cortex and the striatum leads to enhanced processing speed and attention. Some evidence suggests that the stimulants may enhance neuronal recovery after injury.31-33 There are numerous potential side effects with stimulants, including abnormal heart rhythms, decreased seizure threshold, and death, but these severe side effects are extremely rare. The most common side effects with stimulants are decreased appetite, stomach upset, and headache. These are most severe at the beginning of treatment and improve over time for most patients. Insomnia is another common side effect, which may be more frequent in those with a TBI. Amantadine and bromocriptine may also increase dopamine. Studies of these agents have shown reduced abulia, anergia, and anhedonia in those with TBI.34,35 Amantadine may cause confusion, hallucinations, and hypotension. Small studies have suggested some benefits of bromocriptine in cognitive function.36,37 Arousal-enhancing agents also have found a use in the treatment of the neurocognitive sequalae of TBI. Modafinil is the oldest form of these medications, and armodafinil is an isomer of modafinil with longer activity and less side effects. These medications help to increase alertness and wakefulness. The precise mechanism of action of odafinil is unclear. It appears to increase histamine in parts of the brain involved in controlling the sleep-wake cycle; however, knock-out mice that lack histamine receptors still show increased wakefulness with modafinil.38,39 The picture is also murky for modafinil’s effect on orexins, which are wakefulness molecules in the hypothalamus.40 Modafinil has been shown to weakly bind to the dopamine transporter – like the stimulants,41 and dopamine transporter knock-out mice show no response to modafinil.42 A number of research studies have examined the benefit of these agents in fatigue associated with multiple sclerosis, TBI, cancer, and other conditions. Cognitive and memory impairments after TBI may reflect disruption of cholinergic function. The impact of anticholinergic agents on cognitive function of those with TBI supports this contention. Donepezil is the safest and most widely used of the cholinesterase inhibitors. Several easonably large studies have shown improved memory and cognitive function.43-45 Donepezil has benefits in memory and cognition even several years after injury.45,46 Anticonvulsants are often prescribed initially after a TBI due to heightened risk for seizures. Post-TBI mania or mood lability may respond well to anticonvulsants, such as carbamazepine or sodium valproate. They are also often used to treat aggression after TBI. The anticonvulsant agent, topiramate, has been shown to adversely affect cognitive function in the TBI patients.47 While insomnia is a significant issue for patients with TBI, affecting between 15% and 84% (mean of 40%),3,13,19,21,23,48,49 little has been published on the treatment of this aspect of TBI. Benzodiazepines may be effective but carry a risk of disinhibition. Kemp et al48 found that commonly used sleep aid, melatonin, was not effective. Antidepressants, including serotonin reuptake inhibitors and tricyclic antidepressants, are not effective in resolving insomnia in this population.49 No single agent has emerged as a good solution for this symptom. Cognitive rehabilitation Cognitive rehabilitation now takes many forms and is often individualized to the particular needs of the patients. Protocols have been devised to remediate cognitive difficulties often encountered in those with TBI, such as impaired concentration, executive dysfunction, inattention, visual disturbances, memory dysfunction, and impaired language function. They range from simple strategies (using a planner to aid memory and organization) to specific protocols targeting particular cognitive functions (eg, short-term memory) that can be monitored with sequential neuropsychological testing. These interventions have been extensively reviewed elsewhere.50,51 Comprehensive programs which include psychotherapy and social skills components have been shown to have greater efficacy.50,52,53 Overall, reports of benefits have been mixed.54,55 Behavioral therapies Behavioral remediation strategies to eliminate problematic behaviors following TBI have met with mixed success, most often in terms of the poor generalization of specific skills to the outside world. Behavioral deficits that create difficulties for those with TBI and their families include poor hygiene, decline in tidying/cleaning habits, social withdrawal, reduced social comprehension, impaired memory, and poor organization. Behavioral excesses that create difficulties for those with TBI and their families include aggression, sleep disruption, and perseverations. These have been reviewed elsewhere.56 Nutritional supplements Nutritional supplements, herbs, and nootropics have been utilized for many years and are increasingly popular among the patient populations. There remains little clinical research on many of these agents, perhaps reflecting a lack of funding more than a lack of efficacy. Acetyl-l-carnitine is an ester of l-carnitine and is thought to protect brain cells after injury when glucose metabolic pathways are compromised. During this period, acetyll- carnitine supports alternative ketogenic pathways for metabolism.57 It is also believed to enhance cholinergic function. While there are several clinical studies on patients with Alzheimer’s disease and preclinical data on animal models of TBI, the clinical literature on TBI remains sparse. Ginkgo biloba is a natural product of the tree by the same name. It has been shown to improve membrane fluidity and increase resistance to free-radical damage. It provides some subtle benefits to cognitive function in clinical studies of stroke, dementia, aging, and hypoxia damage.58 It has not been systematically studied in TBI but is used extensively in clinic, often in combination with meclofenoxate which is an avid scavenger of free radicals.59 S-Adenosylmethionine (SAMe) is a nutritional supplement which improves cell membrane fluidity and promotes the production of glutathione, an antioxidant. The benefit of SAMe has been assessed in a single clinical study of TBI.60 Patients receiving SAMe had a 77% improvement in clinical scores of post-concussive symptoms. Citicholine provides a source of choline which can cross the bloodbrain barrier. It has been used extensively in Europe and Japan as a treatment for TBI, stroke, and dementia. However, two large US studies failed to demonstrate significant benefit.61,62 Piracetam and the related oxiracetam and phenylpiracetam have shown some promise as nootropic agents. In one double-blind, placebo-controlled study, piracetam improved several symptoms of postconcussive syndrome, including headache and vertigo.63 More recent clinical studies have shown marginal benefit.64 Huperzine-A, an extract of Japanese club moss, is a natural acetylcholinesterase inhibitor. It may serve as a natural alternative to donepezil, rivastigmine, or galantamine. Galantamine warrants special mention as it appears to also modulate nicotinic eceptors and appears to have more persistent benefit in the treatment of Alzheimer’s disease. It appears to modulate neuroimmune responses, in addition to its effects on acetylcholinesterase.65 Cerebrolysin is a polypeptide that purportedly mimics the actions of neurotrophic factors.66,67 Studies have shown that it can reduce beta amyloid and phosphorylated tau protein accumulation. It may promote neurogenesis, synapse formation, and functional recovery.66 In animal models of acute TBI, cerebrolysin-treated rats had more surviving neurons in the area of impact and showed greater functional recovery.67 In a clinical trial of acute TBI, patients were recruited within 24 hours of injury and treated for 3 months with daily intravenous infusion of cerebrolysin. At 3 months, those receiving cerebrolysin performed significantly better on the Cognitive Abilities Screening Instrument.68 It remains unclear if cerebrolysin provides long-term nootropic benefit. The elevation of free radicals in TBI suggests that antioxidants should be beneficial. Clinical trials of pharmacological antioxidants over the past 30 years have not yielded a useful agent in acute TBI.69 Agents, such as tirilazad70 and polyethylene glycol- onjugated superoxide dismutase, have failed to show benefit in acute TBI. Omega-3 fatty acids may enhance brain repair and recovery, based on animal and clinical studies.71 Similarly, vitamin D may offer neuroprotective and restorative benefits72 in the acute TBI setting. In chronic TBI, vitamin D and omega-3 fatty acids may work synergistically, as they both may reduce neuroinflammation, apoptosis, and oxidative stress.73 Other nutritional supplements have been recommended, but prolonged therapy is necessary to possibly see benefits in TBI. A 6-month trial of ginkgo, vinpocetine, acetyl-lcarnitine, huperzine, alpha-lipoic acid, n-acetyl-cysteine, multivitamins, and over 5 g of omega-3 fatty acids daily yielded improved performance in cognitive testing and increased perfusion (function) in single-photon emission computed tomography (SPECT) scan.74 Long-term use of dietary flavanols may improve cognition in mTBI.75 Transcranial magnetic stimulation Transcranial magnetic stimulation (TMS) has shown some promise in animal models of TBI.76 However, a Cochrane review of the clinical application of TMS for depression noted no difference between repetitive TMS (rTMS) and sham rTMS using the Beck Depression Inventory (BDI) or the Hamilton Depression Rating Scale, except during the initial 2-week period.77 The application of TMS in the post-TBI patients is limited by the risk of seizure induction.78 Hyperbaric oxygen Hyperbaric oxygen treatment has been explored as a treatment for TBI.79-91 Hyperbaric oxygen therapy is neither a benign treatment, given the concerns of oxygen toxicity,79 nor a clear treatment in that the placebo condition of moderate hyperbaric room air also effectively improves cognitive function.80,81 The most carefully performed study compared a group in a cross-over design with an interval of both null treatment and hyperbaric oxygen at 100% oxygen and 1.5 atm.82 The study described improvement in many of the symptoms associated with persistent TBI including headache, tinnitus, vision disturbance, memory dysfunction, and impaired cognitive function. Cognitive testing also showed improvement in attention, information processing speed, and a battery of cognitive tests. In an uncontrolled case series of 16 subjects, Harch et al83 demonstrated that an abbreviated series of hyperbaric treatments using 100% oxygen at 1.5 atm could mitigate subjective symptoms of TBI (eg, headache, sleep disruption, irritability), improve cognitive testing scores, and improve cortical function based on SPECT imaging.83 A study of a higher dose (2.4 atm) did not reveal any significant benefit of hyperbaric oxygen therapy compared to a sham-control group treated with 1.3 atm,84 and this result has been extended and confirmed by a related group.85 However, this may reflect an inverse dose- esponse curve, rather than an absence of benefit, in that the low-dose sham group demonstrated significant changes in cognitive testing and symptom frequency.86 Hyperbaric oxygen remains a controversial area in both acute TBI86-89 and chronic TBI.82,83,85,86,90,91 Physical exercise High-energy activities and exercise programs completed through a health club facility or comprehensive rehabilitation program should focus on the same parameters of an age-adjusted and diagnosis-specific program for aerobic conditioning – flexibility, stabilization, and strength. Though it appears safe and is an accepted intervention for TBI, there is a need for further welldesigned studies.92 Exercise was a part of a 6-month study of lifestyle changes described above which yielded improved function based on cognitive testing and perfusion SPECT scans.74
A NEW TREATMENT FOR TBI
Unfortunately, little has been found to reverse the damage of TBI or repetitive concussion which is the root cause of residual cognitive and psychological impairment following TBI.20,93 One potential avenue of treatment for TBI is infrared light, which has shown promising data in a number of applications. Near-infrared (NIR) light has been investigated for its ability to modulate intracellular mechanisms related to healing. The application of NIR light by low-power laser or by light-emitting diode (LED) is also known as laser phototherapy94 or near-infrared photobiomodulation.92 NIR irradiation can facilitate wound healing,95,96 promote muscle repair,95 and stimulate angiogenesis.95,96 NIR phototherapy has been studied and applied clinically in a wide array of ailments, including skin ulcers,97 osteoarthritis,98 peripheral nerve injury,95,96 low back pain,99 myocardial infarction,100 and stem cell induction.101 The finding that NIR light passes relatively efficiently through bone has spurred interest in its application to treating disorders of the brain. Over the past decade, transcranial near-infrared light therapy (NILT)102 has been studied in animal models to understand its ability to repair damaged or dysfunctional brain tissue resulting from stroke and TBI. The first published study of NILT for TBI in humans described two cases of chronic mTBI with significant disability.103 Each patient was treated with an LED device delivering low-level low-level light therapy (LLLT) in the red and NIR range for 6-10 minutes per area daily for several months. Both patients had marked neuropsychological improvement after a minimum of 7-9 months of LLLT treatment. The precise mechanisms underlying photobiomodulation and its therapeutic benefits are not fully understood. The purported effects of NIR are illustrated in Figure 1. Light in the wavelength range of 600-1,200 nm has significant photobiomodulation capability.104 Current data most strongly support that absorption of NIR photons by cytochrome c oxidase in the mitochondrial respiratory chain is the key initiating event in photobiomodulation.95,96,104,105 This induces an increase in cytochrome c oxidase activity which in turn increases adenosine triphosphate (ATP) production. Such an increase in ATP in wounded or underperfused cells may be sufficient to activate cells in areas of injury or metabolic derangement.106 Data from numerous tissue culture and animal studies point to the importance of several secondary molecular and cellular events. For example, NIR photonic energy can modulate reactive oxygen species,95,96,102 activate mitochondrial DNA replication,95,96 increase early-response genes,95 increase growth factor expression, induce cell proliferation, and alter nitric oxide levels.95,96,102 These mechanisms are more fully described in the companion paper.105 When examined in the specific model of neural tissue injury, NIR phototherapy can lead to demonstrable neural repair and recovery. For example, LLLT of a power density of 0.9-36 J/cm2 applied at 24 hours poststroke in a rodent model yielded a 32% reduction in neurological deficits, as well as histochemical evidence of neuron proliferation and migration.106-108 LLLT had similar benefits in a rodent model of TBI.96,109-111 Interestingly, these cellular changes evolved over a period of days after light exposure and persisted for considerably longer than the interval of actual NIR exposure. These findings are consistent with a progressive regeneration cascade set in motion by the NIR light exposure. NILT in stroke NILT, predominately in the form of LLLT, has been investigated in laboratory models of stroke. LLLT applied in a single dose to an ischemic stroke model appeared to induce expression of the growth factor transforming growth factor – beta 1 and suppress the production of peroxynitrite.112 In a rat model of middle cerebral artery occlusion, LLLT at a dose of 0.5-7.5 mW/ cm2 using continuous wavelength light at 808 nm was administered at 24 hours after the acute stroke.108,113 This single application was estimated to deliver 1.8 J/cm2 in total to the cortex surface and resulted in demonstrable neurological improvement. Functional changes were not manifested until approximately 2 weeks after the single treatment. While there was no significant change in the size of the stroke lesion, histochemical evidence of neurogenesis and migrating neurons108 indicate that a cascade of secondary processes was initiated by NILT. A rabbit model of stroke utilizing injection of a blood clot embolus also demonstrated benefit from LLLT.102,114,115 Herein, 808 nm light was applied with an LED delivering 7.5 mW/cm2 and an estimated 0.9-2.6 J/cm2 to the cortical surface. Cortical ATP levels were increased, indicative of increased mitochondrial activity.114 Significant behavioral recovery was also noted; however, neither ATP increased nor neurological function changed at doses less than 0.3-0.7 J/cm2.114,115 At higher doses of 0.9-15 J/cm2, neurological improvement was seen.114,115 The clinical trials of NILT in acute stroke, the Neuro- Thera Effectiveness and Safety Trials 1, 2, and 3 (NEST- 1,-2, -3), were conducted between 2006 and 2009. The Phase II clinical trial (NEST-1) involved 120 patients in a double-blind, placebo- ontrolled study of the effects of NILT within 24 hours of ischemic stroke.116,117 Approximately 60% of the patients experienced clinical benefit, and the safety profile was very good. Thus, NEST-2, a Phase III clinical trial, was undertaken in 2007. A total of 660 patients were enrolled.118 Somewhat surprisingly, the study did not demonstrate statistical clinical improvement using a different outcome measure.119 Post hoc analysis revealed that a portion of the patients who were moderately affected and/or had strokes limited to the cerebral cortex did realize clinically and statistically significant improvement.102 The NEST-3 trial was halted midpoint when it failed to demonstrate statistical benefit on futility analysis.120 A key factor in the interpretation of the results of NEST-3 is that, different from NEST-1, all types of stroke were included as opposed to just cortical strokes. Continuous laser light has a limited depth of penetration (#1 cm into brain tissue) which likely prevents an effect on deeper brain matter. Therefore, the lack of significant benefits from NIR phototherapy in NEST-3 could be related to the fact that ischemic penumbra was not reached by the light (Luis DeTaboada, personal communication, January 2015). While pulsed NIR was not used in the NEST-3 study, it is estimated that pulsed NIR could penetrate up to 3 cm in depth from the cortical surface, therefore possibly extending the therapeutic target to deeper strokes (Luis DeTaboada, personal communication, January 2015). Figure 1 Hypothesized mechanism of action of NiR light therapy. Notes: NiR light (600-980 nm) penetrates tissue to variable depths depending on wavelength, the tissue involved, coherence, and time. A fraction of the photonic energy reaches the mitochondria and is absorbed by cytochrome c oxidase. This activates increased ATP production, increases production of ROS and RNS, and possibly increases NO. Downstream events include increased early-response genes (c-fos and c-jun) and activation of NF-?B, which in turn induces increased transcription of gene products leading to synaptogenesis, neurogenesis, and increased production of inflammatory mediators and growth factors. Abbreviations: NiR, near-infrared; ATP, adenosine triphosphate; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide; NF-?B, nuclear factor kappa B. NILT in TBi Oron et al109 conducted the first animal studies of NILT for TBI. They found that a single application of NIR light at 808 nm from a 200 mW emitter at 4 hours post-injury resulted in a significant reduction in lesion size by 5 days.109 To date, several groups have studied NILT in animal models, and this material has previously been reviewed.95,121-123 Single applications of 800-810 nm NIR light within 4 hours of injury have been shown to improve neurological function significantly.110,124-126 The same dose of NIR light at 6 hours was less effective125 and at 8 hours had no appreciable benefit.125 NIR photonic energy at other wavelengths was less effective. Wu et al110 examined red light (670 nm) at 4 hours and found a similar improvement in neurological function; however, 730 nm and 980 nm had no neurological benefit. Similar data for lesion volume have been reported. A single dose of 800-810 nm NIR light (fluence of 36 J/cm2) yielded an approximate 50% reduction in the volume of the lesion at 3-4 weeks110,111,124-126 and a possible reduction in the initial spread of neurological injury, based on the marked reduction in lesion volume found at 5 days post-injury.109 Repeated NIR phototherapy treatments appear to have some benefit, but the frequency and number of treatments are critical factors. While a single NIR light application had benefit, daily applications for 3 days yielded much greater neurological benefit126,127 with smaller lesion size,126 fewer degenerating neurons,126 more proliferating cells,126 and greater levels of brain-derived neurotrophic factor (BDNF)127 compared to a single treatment in a mouse model. In contrast, daily treatment for 7 days128 or 14 days126 showed no difference from controls. NIR energy densities in the range of 0.9-36 J/cm2 resulted in significant biochemical and behavioral changes.109-111,124-127 Pulsing of NIR light appears to yield a greater neurological response but only within certain parameters. Pulsing at 10 Hz yielded greater neurological improvement and a significant reduction in lesion size compared to either continuous-wave or pulsed NIR at 100 Hz.111 In the mouse model of moderate TBI, NILT (800-810 nm) improved learning and memory (Morris water maze performance),128 as well as behaviors associated with depression and anxiety (immobility during tail suspension).111,124 The finding that NILT brought about a smaller lesion in the rodent model of TBI compared to untreated mice suggests that decreased apoptosis, reduced spreading lesion penumbra, and/or neurogenesis are induced by NILT. Indeed, NILT can decrease BAX expression, a pro-apoptosis gene,129 increase expression of BCL-2, an anti-apoptosis gene,129 increase nerve growth factor,95 increase BDNF,127 decrease inflammatory markers,130 and decrease numbers of degenerating neurons.126 Together, these mechanisms may reduce the enlargement of the initial lesion during the first day following the lesion.109 Moreover, increased BDNF and nerve growth factor may contribute to synaptogenesis as shown by increased levels of synapsin-1,127 and neurogenesis, as shown by increased numbers of proliferating cells.127 In a double-blind study in healthy volunteers, NILT was beneficial – compared to sham – in memory and attention.131 In this study, the authors shed only one application of NIR light to the right forehead, targeting the right frontal pole of the cerebral cortex (Brodmann’s area 9 and 10). The device was a Class IV laser CG-5000 (Cell Gen Therapeutics, Dallas, TX, USA), and the parameters were as follows: wavelength 1,064 nm, irradiance 250 mW/cm2, fluence 60 J/cm2, and time 4 minutes per site (two sites).131 The subjects who received the NIR treatment had better attention after 2 weeks, measured by the psychomotor vigilance test. They also had better delayed visual memory at the Delayed Match-to-Sample test. This is the only published controlled trial assessing the impact of NILT on cognition; however, other reports have shown the therapeutic effects of NILT in small numbers of TBI patients. In a two-case report in TBI patients,103 NILT (870 nm) improved sustained attention, memory, and executive functions. Both patients were treated with an instrument with three separate LED cluster heads. The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 2.2-25.8 mW/cm2, fluence 13.3 J/cm2, and time 10 minutes per site.103 The same group reported on a cohort of eleven subjects with persistent cognitive dysfunction and treated with a similar NILT protocol for chronic mTBI.132 The eleven subjects received NILT with a device with three LED cluster heads (Model 1100; MedX Health, Toronto, ON, Canada). The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 22.2 mW/cm2, fluence 13 J/cm2, and approximate time 10 minutes per site. The NIR light was applied three times per week for 6 weeks (18 sessions), on eleven sites for 10 minutes per site (the total duration of each session was 20 minutes).132 The sites on the skull were chosen on the midline, and bilaterally on frontal, parietal, and temporal areas. At the follow-up neuropsychological testing, NILT had a powerful effect on attention, inhibition, and inhibition switching in the Stroop task, and similarly improved verbal learning and memory, as well as enhanced long-delay free recall on the California Verbal Learning Test. Eight subjects, from the same cohort, were identified as having mild, moderate, or severe depression based on the BDI-II total score (range: 15-34).132 The three cases, who entered the study with only mild depression, remained the same after NILT treatment. Results for the five cases with moderate-severe depression were as follows: two moderate cases improved to mild/minimal depression 8 weeks after the end of NILT series, and one severe case improved to moderate depression. Two moderate or severe depression cases remained the same after 8 weeks of follow-up from the last NILT session.132 Dose response and photonic penetration A prevailing theory in photobiomodulation postulates that a bimodal response curve exists for the biological effects of NIR light.95 The so-called Arndt-Schulz curve (a fundamental principle in homeopathic medicine) is frequently used to describe this biphasic dose response. Some data indicate that low levels of light have a much better effect on stimulating and repairing tissues than higher levels of light. Laboratory studies of cells in culture have demonstrated a bimodal dose response to light exposure in lymphocytes133 and fibroblasts.134,135 For example, Chen et al135 found that a range of 0.03-0.3 J/cm2 was beneficial in activating transcription factors in culture, while 3-30 J/cm2 inhibited the activation of these factors. In contrast, an order-of-magnitude greater dose (2 J/cm2) was best at activating fibroblasts in a superficial wound model.136 Furthermore, an order-ofmagnitude greater dose (30 J/cm2) proved to be best in a rodent joint inflammation model.137 Thus, a dosedependent effect for many biological responses to NIR light has been demonstrated,95,137-139 but the critical parameter is dose at the level of the target tissue, rather than at the surface.137,140 The amount of energy that reaches a volume of tissue at depth is determined by the attenuation of the photonic energy as it passes through the overlying tissue. For example, only 2.45% of the energy from a 980 nm laser emitter penetrates to the level of the peroneal nerve.140 Nevertheless, the biphasic dose response does not appear to be universally true. In primary microglial cell culture, a dose-dependent response to NIR was demonstrated with no detrimental effects at doses as high as 30 J/cm2.141 So a critical question in the use of NILT is that of radiant energy penetration. In particular, some authors have challenged the efficacy of low-power LEDs used in LLLT.142-144 In laboratory studies, LLLT radiant energy is almost entirely absorbed in the first 1 mm of skin.145,146 In two unrelated studies, LLLT diode devices proved to be ineffective in the treatment of diabetic neuropathy,142,144 in contrast with prior reports.147 Similarly, laboratory studies of NILT using LLLT transcranially have not consistently yielded positive results. For example, in a rat model of TBI, Giacci et al148 found no benefit from daily 30-minute irradiation with either 670 nm or 830 nm 0.5 W LED emitters for a period of 7 days. Doses at the skin surface were 28.4 J/cm2 and 22.6 J/cm2, respectively.148 Similarly, treatment of a rat model of contusive spinal cord injury with LLLT (830 nm at 22.6 J/cm2 or 670 nm at 28.4 J/cm2) for 30 minutes per day for 5 days resulted in no significant functional improvement and no reduction in lesion size, despite delivering 2.6 J/cm2 to the spinal cord.148 Lapchak102 reported that the physical parameters of NILT in the clinical trials for the treatment of stroke utilized in the NEST-1 and NEST-2 trials116-120 may have delivered insufficient energy to cortical tissues to be effective. Therein, NIR light of 808 nm wavelength with infrared energy densities of 0.9 J/cm2 was applied to the human scalp for a total of 40 minutes with applications at multiple sites during that time.116,118 Recall that animal models of both stroke and TBI suggest that NIR energy densities in the range of 0.9-36 J/cm2 resulted in significant biochemical and behavioral changes.96,106-115,125-127 The concern raised from the NEST studies102 is that current clinical trials testing the effectiveness of lowenergy NIR diodes to treat TBI may yield negative or inaccurate efficacy data, not because of a failure of infrared light to invoke a change but due to a dose error. Doses that are effective when directly applied to cells in a Petri dish149,150 or to 3-5 mm thick rodent brains96,109-111,125,126,128 may be insufficient to penetrate 2-4 cm into the human brain. In a companion paper, our own studies of photonic energy penetration are detailed.105 To summarize, the laboratory tissue studies showed that 0.5 W LED emitters did not penetrate the 2 mm thickness of human skin. No detectable energy from 0.5 W LED NIR light emitters could be detected penetrating a similar thickness (1-2 mm) of sheep skin or 3 cm thick section containing sheep skin, skull, and brain. In contrast, 11% of the photonic energy from a 10 W 810/980 nm coherent NIR laser penetrated 2 mm of human skin. Similarly, 17% of the photonic energy from a 15 W 810 nm coherent NIR laser penetrated the same distance.105 Energy from these more powerful NIR emitters could be detected penetrating 3 cm of sheep skin, skull, and brain with 0.4% of the 10 W 810/980 nm NIR laser’s energy reaching the depth of 3 cm and 2.9% of the 15 W 810 nm NIR laser’s energy traversing the same distance.105 Anders also has demonstrated penetration of 808 nm light to 40 mm in the brain using a 5 W laser emitter (JJ Anders, personal communication, January 2015). Prompted by the mixed results in the literature and the observations by Lapchak,102 Franzen-Korzendorfer et al,144 Wan et al151 and Lavery et al142 we have been utilizing relatively high-power (10- 5 W) lasers at the wavelengths of 810 nm and 980 nm in the clinic to treat patients with TBI. Clinically, the patients have shown excellent responses with resolution of many of their long-standing symptoms of TBI or post-concussive syndrome. Below is a retrospective series of such patients to illustrate the extent and character of response to this modality. Methods Patients in the case series were sequentially treated patients at a clinic which is engaged in ongoing NILT for a number of clinical conditions. The risks, benefits, and current state of research on the use of NILT were explained to each patient. Each patient consented to treatment. Institutional Review Board approval was obtained in a post hoc review, noting that the risk-benefit ratio was acceptable. Between March 16, 2011 and February 20, 2013, sequential new referrals for chronic mild-to-moderate TBI were evaluated for treatment and selected for NILT using Class IV lasers, either the LT1000 (LiteCure, Newark, DE, USA), a 10 W adjustable NIR laser emitter with wavelengths of 810/980 nm capable of delivering continuous or pulsed NIR light, or the Diowave 810 (Diowave, Riviera Beach, FL, USA), an adjustable NIR emitter up to 15 W with a wavelength of 810 nm capable of delivering continuous or pulsed NIR energy. Demographics and laser treatment settings are detailed in Table 1. The fluence delivered to the skin of patients ranged from 55 J/cm2 to 81 J/cm2. No other treatment modalities (medications, exercise regimen, supplements) were added, discontinued, or changed while receiving NILT. Symptoms were monitored clinically. A baseline Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)152 was completed for all patients, and the BDI153 was administered to seven of the ten patients before and after the course of treatment. In addition, each patient was instructed on how to create and maintain a patient and spousal diary of symptoms and subjective progress. Each of six patients received a single series of ten treatments with the LT1000 Class IV laser. Three additional patients each received a single series of 20 treatments with the LT1000 Class IV laser. One patient was treated with the Diowave 810 nm Class IV laser device in a series of 20 treatments. The patients and treating clinician wore protective eyewear. There were no incidents of burns or thermal discomfort (Figure 2). The impact of high-watt NILT While the patient group represented a diverse mix (Table 1 presents demographics), some notable commonalities of symptoms emerged. Over 90% of the patients had complaints of anxiety, depression, irritability, and insomnia. Other symptoms included headache (60%), suicidal ideation (50%), cognitive difficulties (50%), attention problems (50%), short-term memory problems (40%), loss of libido (30%), substance abuse (20%), fatigue (20%), and panic attacks (20%). Six of the patients were unemployed prior to treatment. Three of the patients were experiencing severe marital difficulties. All carried or had a confirmed diagnosis of TBI, but other comorbid diagnoses included PTSD, major depressive disorder, generalized anxiety disorder, bipolar disorder, and attention deficit/hyperactivity disorder. The patients’ baseline scores on the BDI were 25.3±12.1 (moderate depression range), and baseline scores on the QIDS-SR were 12.9±4.6 (moderate depression range). During NILT treatments, skin temperature increased no more than 3°C with rapid cooling after removal of the NIR light. A continuous sweeping motion was utilized to minimize skin heating and cover a larger area. After a course of ten treatments of NILT (20 treatments in four patients), each patient experienced significant clinical improvement with resolution of many of their symptoms (Table 2). In addition, the BDI scores dropped to 12±6.5 (nondepressed range). This represented a significant decrease (P,0.01, Student’s t-test, one-tailed, Microsoft Excel). The QIDS-SR scores after treatment were 2.2±2.3 (nondepressed range), and the difference from baseline was highly significant (P,0.00001, Student’s t-test, one-tailed). Patients noted improvement in cognitive function, mood, anxiety, and sleep. None of the patients continued to have suicidal thoughts (50% at baseline). Other symptoms, such as anxiety and irritability, were markedly improved. Most notable were the nonquantifiable changes in patients’ lives. Patients reported improved cognitive ability and a desire to return to meaningful work. Five of the six unemployed patients have returned to work. The two patients who were Iraq/ Afghanistan veterans have found new careers in highly skilled trades. The patients with marital difficulties have reconciled and were purchasing homes or otherwise solidifying their marriages. The clinical change can be attributed to NILT because no changes in medications, supplements, or exercise regimen were permitted during the course of NILT treatment. All patients in the case series experienced significant clinical improvement which supports the conjecture that high-power NIR laser delivers sufficient energy to the human brain for photobiomodulation to occur. Insomnia and suicidal ideation, common symptoms in those with TBI or post-concussive syndromes,3,17-20,24,25 resolved in 100% of cases. Headache, another common symptom for patients following a TBI,6,14,15,23 was reduced or resolved in the six patients so afflicted. Symptoms such as anxiety,14,15,21,24 depression,21,24,25,27-29 and irritability resolved or were dramatically reduced in all patients. Cognitive function appeared to improve based on return to work or improved work performance, although cognitive tests were not performed. The quality of life dramatically improved in all cases, based on the observations of the patients, their family members, and the treating clinician. At follow-up intervals of 6-7 months post-treatment, patients have reported continued improvements in symptoms. The precise areas of brain injury were not elucidated in Figure 2 Treatment parameters per individual, based on area of the skull treated. Notes: Dimensions varied per head/skull size and hair line. Treatment was warm and comfortable for each patient. There were no incidences of discomfort. Areas treated were (A) temporal- ilateral, (B) frontal, and in patients 1-3, 5, and 6 (B) frontal only. Table 1: Infrared light treatment parameters for each of the ten patients in the case series Patient Area treated Sex Mechanism of TBI Interval since TBI Wavelength of Dosage per area Duration before treatment NIR-PT dual wave Scanning technique per area pulsed 10 Hz 1 B, bilateral frontal Male Concussive blast 2 years 810 and 980 nm 2,700 J 10 minutes Fluence – 20.45 J/cm2 2 areas Area – 132 cm2 10 visits 2 B, bilateral frontal Female MVA 18 years 810 and 980 nm 2,400 J 9 minutes Fluence – 18 J/cm2 2 areas Area – 133 cm2 10 visits 3 B, bilateral frontal Female MVA 5 years 810 and 980 nm 2,400 J 8 minutes Abuse Fluence – 18.3 J/ cm2 2 areas Area – 131 cm2 10 visits 4 A–B, bilateral frontal, left temporal Female MVA x2 8 years and 13 years 810 and 980 nm 2,400 J 8 minutes Fluence – 18.3 J/cm2 3 areas Area – 131 cm2 10 visits 5 B, bilateral frontal Male Vietnam Veteran 20+ years 810 and 980 nm 3,000 J 10 minutes Concussion Fluence – 28.3 J/cm2 2 areas Child abuse Area – 106 cm2 10 visits 6 B, bilateral frontal Male Concussion 5+ years 810 and 980 nm 2,400 J 12 minutes Fluence – 14.8 J/cm2 2 areas Area – 162 cm2 10 visits 7 B–A, bilateral frontal, left temporal Male Afghanistan, Iraqi Disability 810 and 980 nm 3,000 J 10 minutes Disability due to TBI 2 years Fluence – 22.7 J/cm2 3 areas Area – 132 cm2 20 visits B–A, bilateral frontal, bilateral temporal Female Hypoxic encephalopathy Childbirth-related 810 and 980 nm 2,700 J 9 minutes injury, 8 years Fluence – 27.8 J/cm2 3 areas Area – 97 cm2 20 visits 9 B–A, bilateral frontal, bilateral temporal Male MVA-TBI Numerous episodes 810 and 980 nm 3,000 J 10 minutes Concussions Fluence – 22.72 J/cm2 3 areas Area – 132 cm2 20 visits 10 B–A, bilateral frontal, left temporal Female Bicycle vs car >30 days 810 nm single 2,700 J 9 minutes Concussion, amnesia, LOC wavelength – Fluence – 17.1 J/cm2 3 areas different device Area – 158 cm2 20 visits Note: All safety precautions were followed, including metal protective eyewear (laser eye protection). Abbreviation: LOC, loss of consciousness; MvA, motor vehicle accident; TBi, traumatic brain injury. the majority of these cases, so a correlation of symptoms changes and cortical function changes cannot be made; however, perfusion SPECT imaging in other patients has shown significant increases in perfusion in injured areas of the brain and overall improved cortical function following similar courses of high-watt NILT.154 One concern that has been expressed about high-watt NIR lasers is the risk of tissue heating.155 We explored this issue in our companion paper on NIR penetration.105 Temperature change was 1°C-3°C at the skin surface using continuous-wave NIR lasers in the range of 10-15 W. Using pulsed settings, the high-powered lasers showed no significant temperature change in tissue samples. The temperature change on human skin was 1°C or less in the in vivo penetration studies while maintaining continuous movement of the laser probe head.105 Clinically, patients in this case series reported only slight warming of the skin, but no discomfort, using the continuous motion technique. Laboratory studies have largely focused on treatment of acute brain injury. The processes involved in the benefits of NIR light in chronic TBI as seen in this clinical case series may be quite distinct. Nevertheless, Schiffer et al156 found that a single application of LLLT at 810 nm and 250 mW to the forehead over 8 minutes reduced depression and anxiety symptoms in ten patients for approximately 2 weeks. Similarly, the small case series by Naeser et al103 demonstrated some benefit using NIR light, albeit at very low power levels over a prolonged course of several months with only transient benefit. Together with our clinical data, these findings suggest that at least some of the photobiomodulatory effects of NIR energy likely do occur in chronic neurological conditions. Prior presentations on NILT for the treatment of TBI or stroke in humans have focused on getting photonic energy through the skull to the cortex surface which traverses a distance of about 6-10 mm; however, this model is flawed in that the distance to the areas of damage may be far greater. In other words, the cortex immediately subjacent to a portion of the skull may be 10 mm from the surface, but the NIR light energy may need to penetrate 3-7 cm to reach areas of damage. Much of the cortical surface is actually lining the walls and floors of sulci, rather than immediately subjacent to the skull. Analysis of NIR spectroscopy reveals that light propagation through varying media with irregular boundaries is subject to high levels of scatter.157 In addition, review of the neuroimaging literature on TBI has revealed that the most common areas injured in TBI are the orbitofrontal cortex (at the ventral surface of the frontal lobe) and the anterior and medial temporal lobes.158 It is not anatomically possible to position an NIR light emitter immediately exterior to the skull overlying these areas. Indeed, the orbitofrontal cortex positioned immediately above the eyes can only be reached from the forehead by angling the light emitter. Similarly, the temporal lobes are separated from the surface by epidermis, dermis, subcutaneous fat, subcutaneous blood vessels, accessory head of the temporalis muscle, connective tissue, temporalis muscle, skull, and dura mater.159 Each of these structures has different absorption and refraction properties, and each interface between different materials also creates a barrier to transmission of photonic energy.157 Blood flowing in the subcutaneous vessels is believed to create a unique barrier to transmission.160 In summary, effectively targeting the areas most commonly injured in TBI with sufficient photonic energy to initiate reparative processes represents a significant challenge in NILT. This appears to have been overcome with the high-power laser protocol presented here and in a related paper.154 As yet, the mechanism of action of NILT in treating TBI is not entirely clear. Moreover, the neurological benefits are not immediately apparent. Rather, a delay of 1-4 weeks was noted, consistent with a progressive regeneration cascade set in motion by the NILT.96,103,105 ,107,109,121,122,124,127,135 Similarly, most of the patients in the present case series did not notice benefits immediately or within the first few treatments. Instead, they reported benefits emerging over an interval of weeks, and in some cases, continuing after completion of the course of NILT. In addition, the clinical improvement reported by the patients in the above case series is more profound than that reported by patients treated with LLLT or low-powered lasers.103 In fact, we observed that among seven subjects with documented moderate depression, per BDI scores, four had an antidepressant response (≥50% decrease of depression severity). In contrast, Naeser et al132 reported that out of eight subjects with TBI and comorbid depression, only three had a significant improvement in their depressive symptoms (37.5%). Our results may be due to the greater penetration of more powerful, coherent, and pulsed NIR light from a laser source. A unique outcome measure was developed for this protocol (Morries and Henderson, unpublished data, 2015). A patient diary and separate spousal diary provided a weekly update of patient’s response in his or her home environment. This novel approach to capturing the patient treatment experience provided the patient and family with tangible and pertinent documentation of the clinical response. While time consuming, the experiences recorded in these diaries proved to be valuable clinical tools to the treating clinicians.
CONCLUSION
To date, there has been little progress in developing effective treatments for chronic mild-to-moderate TBI or repetitive concussions. This area of need has become even more pressing with the return of veterans from military conflicts in Iraq and Afghanistan4,6,7,16,17,19,161 and the recognition of the magnitude of sport-related TBI.5,8-10 In addition, the dramatic growth in the geriatric population with attendant proprioceptive dysfunction has resulted in a rising incidence of fall-related TBI.162 NILT has shown promise as a tool for the treatment of TBI. A critical issue is to assure that adequate photonic energy reaches the injured areas of the brain. The use of high-wattage lasers, as we have demonstrated, results in marked clinical improvement in patients with chronic TBI. Moreover, symptoms consistent with PTSD, anxiety, and/or depression also improved considerably or resolved in this group of patients. Further work in the use of highwattage NILT in the treatment of TBI, depression, and other neurological disorders is encouraged.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Mr Charles Vorwaller (Aspen Lasers) and Lite Cure Corporation. The authors also acknowledge the contribution of Ms. Taylor Tuteur in the artistic creation of Figure 1.
DISCLOSURE
Dr. Larry D Morries is the CEO of Neuro-Laser Foundation, a nonprofit foundation. He has a private practice in Lakewood, CO. Theodore A Henderson is the president of The Synaptic Space, a medical consulting firm. He is Table 2 NiLT case series with demographics, symptoms, and treatment response
PRETREATMENT POSTTREATMENT
Patient # Sex Occupation Mechanism of TBI Diagnoses Sleep Symptoms Suicidal BDI Sleep Symptoms Suicidal BDI 1 M Veteran, Blast – 5 years; TBI, PTSD, MDD Primary and H, S, I, D, X, L, A, M, + – Resolved None, back No – unemployed Iraqi middle C, SL with spouse, insomnia working 2 F Nurse, MVA – 8 years TBI, PTSD Middle and H, F, I, X, C, A, STM, L, + 18 Resolved A and HA – No 15 unemployed terminal HA, SL but mild, insomnia return to work 3 F Unemployed Assault and TBI, PTSD, MDD, Primary and D, X, P, M, L, HA, S, + 23 Resolved HA – mild, No – MVA, 5 years GAD, ADHD middle insomnia, SA, C, N, STM back with Prior nightmares spouse, no SA, working 4 F Unemployed MVA – 3 years, TBI, PTSD, MDD Primary and D, X, HA, I, M, SA, S, N + 23 Resolved None, marriage No 17 assault middle insomnia, improved, numerous violent nightmares no SA, working 5 M Veteran, Blast – 20+ years TBI, MDD, GAD Primary and D, X, I, S, SL + 18 Resolved None No 1 unemployed 1960s; Vietnam middle insomnia 6 M executive Trauma – TBI, GAD, MDD Primary D, X, I, P, HA, A, S – – Resolved HA, X, and P – No – chronic insomnia but improved 7 M Veteran, Multiple blasts TBI, MDD, GAD Primary and S, D, I, X, C, A, S, STM, – 22 Resolved HA and C – No 16 disability (>12); Afghan middle HA mild, new and Iraqi wars insomnia career 8 F Student Childbirth TBI, learning Primary D, I, X, C, A, SL, F, STM – 16 Resolved, STM improved, No 7 disorder insomnia no bads reading .20% dream more animated 9 F Sales MVA and TBI, LOC Primary and HA, SL, N, D, I, X, H, A – 29 Resolved Mild HA, No 9 sports TBI middle insomnia, job nightmares promotion 10 F Physicist Recent car– TBI, LOC, amnesia Primary and D, I, X, neck, knee pain – 51 Resolved No loss No 19 bicycle middle of skills, accident insomnia maintain intellectual job Notes: Demographics for each of the ten patients in this case study is presented. Also presented is their history of mechanism of injury, diagnosis, and related symptoms. Changes in anxiety levels, sleep patterns, depression, and suicidal ideation were important symptoms and outcomes to track. Patients were instructed for no medication changes, with their primary treatment provider’s approval. Cognitive difficulties, attention problems, and short-term memory difficulties were by patient interpretation of their symptomatic improvement and patient diary changes. Symptom occurrence % was as follows: Anxiety – 100%, Depression – 90%, Irritability – 90%, Primary And Middle Insomnia – 90%, Headache – 60%, Sadness – 60%, Suicidal Ideation – 50%, Cognitive Difficulties – 50%, Attention Problems – 50%, Short-Term Memory Problems – 40%, Marital Difficulties – 30%, Loss Of Libido – 30%, Substance Abuse – 20%, Fatigue – 20%, Panic Attacks – 20%. Abbreviations: NILT: Near-Infrared Light Therapy, TBI: Traumatic Brain Injury, PTSD: Post-traumatic Stress Disorder, MDD: Major Depressive Disorder, GAD: General Anxiety Disorder, ADHD: Attention Deficit/Hyperactivity Disorder, H: Hyperarousal, S: Sadness, I: Irritability, D: Depression, X: Anxiety, L: Loss Of Libido, A: Attention Problems, M: Marital Difficulties, C: Cognitive Problems, SL: Sleep Issues, F: Fatigue, STM: Short- erm Memory Problems, HA: Headache, P: Panic Attacks, SA: Substance Abuse, N: Nightmares, BDI: Beck Depression Inventory, LOC: Loss of Consciousness, MVA: Motor Vehicle Accident. the president of Dr. Theodore Henderson, Inc., a clinical service firm. He is the co-owner of Neuro-Luminance, a clinical service organization. He is the president of the International Society of Applied Neuroimaging. He is the CFO of the Neuro-Laser Foundation, a nonprofit foundation. Dr. Paolo Cassano received funding from the Brain and Behavior Research Foundation; Photothera Inc and from the Dupont Warren Fellowship (Harvard Medical School) to conduct research on NIR light for the treatment of major depressive disorder.
ABOUT THE AUTHORS:
Larry D. Morries, DC brings a distinguished 30-year career studying and treating the brain and body through his private practice based in Lakewood, Colorado. As Neuro-Laser Foundation’s co-founder, his chiropractic expertise is complemented with extensive study of near infrared-light therapy applications, clinical radiology, clinical neurology and sports injury and rehabilitation. In practice since 1973, Dr. Morries has contributed extensively to both chiropractic and medical professions throughout his career. He is a recognized expert often called upon for review services, treatment utilizations, and documentation presentations. In recent years, he has guided the Colorado State of Colorado Workers Compensation Board with a review of treatment guidelines for Chronic Pain, and Complex Regional Pain Syndrome, Shoulder Pain, Low Back Pain, Traumatic Brain Injury, and was asked to present in 2016 on Thoracic Outlet Syndrome.
Other professional involvement include:
• Colorado Chiropractic Association, Board member, President in 1982, Chairman in 1984
• Colorado Chiropractic Society, Vice President and Secretary in 1995-2004
• Colorado Chiropractic Journal Club, Chairman,since 2008
Dr. Morries has continued his study of the human body and brain with postgraduate work in Neurodiagnostic testing at the American Academy of Neurology, and Harvard Medical School-Massachusetts General Hospital. He is also educated on Spinal Mechanics at Chicago Rehabilitation Institute. He earned his Doctorate in Chiropractic from Logan Chiropractic College, with recognition as Student Clinical Director, Teaching Assistant in Radiology. Dr. Morries is most proud of his research papers and awards, in America Academy of Pain Medicine, Sciatic and Suprascapular Nerve Blocks with Dr. Steve Gulevich, MD. He was asked to share two Poster presentations at the North American Laser Foundation in 2011on Low Back Pain, plus Polyneuropathy treatment with Laser (NIR) therapy. His Podium Presentation and publication on Hip dysplasia, in American Board of Chiropractic Sports Physicians®. Additionally, he has given presentations abroad at State of Chiropractic Research, Foundation of Chiropractic Education and Research, in Bournemouth England and Vancouver, BC, Canada. Dr. Theodore Henderson has extensive training and experience to the practice of Psychiatry. He trained in Psychiatry at the prestigious Barnes/Jewish Hospitals at Washington University in St. Louis. Dr. Henderson completed a fellowship in Child & Adolescent Psychiatry at the University of Colorado. He also has training in Radiology, Nuclear Medicine, and the genetics of psychiatry. He established his private practice in Centennial Colorado in July of 2000. Dr. Henderson brings a unique blend of expertise in psychopharmacology, neurobiology, and an understanding of human nature to the practice of psychiatry. Dr. Henderson attended medical school at Saint Louis University School of Medicine. While in medical school, he began studying heart pathology under Dr. Vernon Fischer. He earned an American Heart Association Medical Student Research Fellowship. With this fellowship, he spent one year at the University of Washington studying the pathology of atherosclerosis. In 1991, Dr. Henderson founded the Child Abuse Prevention Task Force at Saint Louis University. This program taught children, parents, and teachers about child sexual abuse and how to prevent it. Each year, this program reached over 8,000 children throughout the metro St. Louis area, primarily in the poor inner-city schools. The program was awarded numerous awards, including a Saint Louis University Community Service Award, Commendations from the school districts, and an award from the American Medical Student Association. Dr. Henderson was nominated for a Student Life Leadership Award and earned a Departmental Award from the Department of Community and Family Medicine. He also received a Weis Humanitarian Award recognizing outstanding humanitarian care as a medical student. Dr. Henderson wrote a training manual on this program that was implemented at other medical schools and he cowrote a book chapter in the book, A Parent’s & Teacher’s Handbook on Identifying and Preventing Child Abuse (1998). During graduate school and medical school, Dr. Henderson published numerous research studies. He published 9 articles and 27 abstracts about his research in brain development. He also published a book chapter on brain development in collaboration with his research professor, Dr. Mark Jacquin. His research focused on the role of neural growth factors and impulse activity on the development of brain organization. He collaborated with leading researchers, including Drs. Thomas Woolsey, Eugene Johnson, and Thomas Rhoades. While a medical student, Dr. Henderson wrote two research grants (as part of program project grants). Both were funded. He continued conducting research at Saint Louis University and Washington University throughout his residencies. Dr. Henderson trained for one year in Radiology, focusing on neuroimaging and pediatrics. With this strong base, he then undertook a residency in Psychiatry at Washington University’s program at Barnes/Jewish Hospitals in St. Louis. His residency included extended training in general pediatrics at St. Louis Children’s Hospital. In 1997, He was awarded the National Institute of Mental Health Outstanding Resident Award for his ongoing work in child abuse prevention and his neurobiological research while a resident. Dr. Henderson completed a residency in Adult (or General) Psychiatry and then undertook a fellowship in Child Psychiatry at the University of Colorado. This included additional specialization in Autism and Autism Spectrum Disorders. He compl
Effect of autologous mesenchymal stem cells induced by low level laser therapy on cardiogenesis in the infarcted area following myocardial infarction in rats
Hana Tuby1, Tali Yaakobi1, Lidya Maltz1, Yaakov Delarea2, Orit Sagi-Assif2, Uri Oron1* - (Publication) 4467
This study showed rats that were give a heart attack and then treated with the laser on their shins saw a 55% reduction in infarction size in the heart showing that the stem cells released from the bone migrated to the heart.
View Resource
1Department of Zoology, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv, Israel
2Department of Cell Biology and Immunology, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv, Israel
Email: *oronu@post.tau.ac.il
Received 27 May 2013; revised 29 June 2013; accepted 16 July 2013
ABSTRACT
In this study, we investigated the hypothesis that photo- biostimulation by low-energy laser therapy (LLLT) applied to the bone marrow (BM) of myocardial in- farcted rats may attenuate the scarring processes that follow myocardial infarction (MI). Wistar rats under- went experimental MI. LLLT (Ga-Al-As diode laser) was applied to the BM of the exposed tibia at differ- ent time intervals post-MI (4 hrs, 48 hrs and 5 days). Sham-operated infarcted rats served as control. In- farct size was significantly reduced (55%) in the la- ser-treated rats as compared to the control non-treat- ed rats, at 2 weeks post-MI. A significant 3-fold in- crease was observed in the density of desmin immu- nopositive stained cells 14 days post-MI in the infarc- ted area of the laser-treated rats as compared to the non-laser-treated controls. The electron microscopy from the control infarcted rat hearts revealed a typi- cal interphase area between the intact myocardium and the infarcted area, with conspicuous fibroblasts with collagen deposition dispersed among them. In rats that were laser treated (to BM), the interphase zone demonstrated cells with different intracellular struc- tures. There was also a significant increase in the per- centage of c-kit positive cells and macrophages in the circulating blood of the laser treated rats as compar- ed to control non treated ones. In the majority of the cells clusters of myofibrils anchored to well-developed Z-lines and structures resembling the morphological characteristics of mature intact cardiomyocytes were evident. In conclusion, LLLT to the BM of rats post- MI induces cardiogenesis mainly at the borders of the infarcted area in the heart.
Keywords: Low-Level Laser Therapy; Myocardial Infarction; Macrophage; Desmin; Ultrastructure; c-Kit Positive Cells
1. INTRODUCTION
Regenerative capacity and mitotic activity in the heart are confined mainly to the lower vertebrates [1]. Amputation of ~20% of the zebrafish’s ventricular myocardium re- sulted in full regeneration without scarring [2]. In am- phibians, heart injury was associated with increased cell proliferation of myocytes and enhanced regeneration [3]. The adult mammalian heart was traditionally considered to be a post-mitotic organ with terminally differentiated cardiac myocytes. However, this dogma has recently been challenged by several studies and reviews [4-8]. These studies have suggested that cardiac myocytes are replaced throughout the lifespan even in the human heart, and that myocytes can regenerate from resident cardiac progenitor cells (CPC) as well as from bone marrow (BM). Studies in human infarcted hearts have shown evidence of cytoki- nesis of cells in the heart and evidence of cardiac stem cells that are activated in response to ischemic injury. This growth response is attenuated in chronic heart fail- ure [9]. Some studies have reported that cardiac myocyt- es can be derived from BM; specifically, side population precursor cells following induction of myocardial infarc- tion (MI) by left anterior descending artery (LAD) liga- tion [10-12]. Contradicting these findings, other laborato- ries using genetic markers have reported that lineage ne- gative, c-kit+ BM cells did not differentiate into cardio- myocytes [13]. It was also suggested that BM-derived stem cells may stimulate the small population of stem cells in the ischemic heart to proliferate and differentiate to enhance cardiac repair post-MI [14]. In a recent study transient regenerative potential in the mouse heart was demonstrated during the neonatal period [15].
Low-level laser therapy (LLLT) has been found to modulate various biological processes [16,17], such as increasing mitochondrial respiration and ATP synthesis [18], facilitating wound healing and promoting the proc- ess of skeletal muscle regeneration and angiogenesis [19- 21]. In an experimental model of the infarcted heart in rats and dogs, it was demonstrated that LLLT application directly to the infarcted area in the heart at optimal power parameters significantly reduced scar tissue formation [22-24]. This phenomenon was partially attributed to a significant elevation in ATP content, heat shock proteins, vascular endothelial growth factor (VEGF), inducible ni- tric oxide (NO) synthase, and angiogenesis in the ischemic zone of the laser-irradiated rats, as compared to non- irradiated rats [25].
The effect of photobiostimulation on stem cells or pro- genitor cells has not been extensively studied. LLLT ap- plication to normal human neural progenitor cells signi- ficantly increases ATP production in these cells [26]. LLLT delivery to MSCs and cardiac stem cells in vitro caused a significant enhancement in their proliferation rate [27,28]. LLLT has also been shown to increase the proliferation rate of adipose-derived stem cells in vitro [29]. Recently, we demonstrated that LLLT application to autologous BM could induce mesenchymal stem cells (MSCs) in the BM to proliferate and cause their recruit- ment and specific homing in on the infarcted rat heart and not on other organs [30,31]. The laser treatment to the BM also caused a marked and statistically significant reduction of 79% in the scarring and ventricular dilata- tion followed MI as compared to infarcted non-laser- treated rats. The aim of the present study was to investi- gate the possibility that induction of stem cells in the BM of rats by LLLT could also affect cardiogenesis in the in- farcted rat heart.
2. MATERIALS AND METHODS
2.1. Experimental Procedures
A total of 21 Wistar male rats, weighing 200 - 250 gr, that underwent ligation of the LAD artery to induce MI, were used as described by us previously [23]. All the ex- perimental procedures were approved by the animal care committee of Tel-Aviv University. Briefly, rats were anes- thetized with Avertin (1 ml/100 g body weight I.P.) and the lungs were ventilated. Thoractomy was performed by invasion of the intercostals muscles between the 5th and 6th rib to expose the heart. The LAD artery was occluded 2 mm from the origin with 5-0 polypropylene thread (Ethicon Inc., Cincinnati, OH). Following LAD artery occlusion the chest muscles and skin were sutured and the rats were ventilated until they woke up. The infarcted rats were divided randomly into two groups. In one group LLLT was applied directly to the BM 4 hrs, 48 hrs and 5 days post-MI (see below). The second group was non-laser-treated (the rat’s bone was exposed for the same duration as the laser-treated group but the laser was not turned on). Food and water were supplied ad libitum. Rats were sacrificed 14 days post-MI.
2.2. Laser Application
After induction of MI rats were randomly assigned to a laser-treated or control non-laser-treated group. A diode (Ga-Al-As) laser, wavelength 804 nm with a tunable po- wer output of maximum of 400 mW (Lasotronic Inc., Zug, Switzerland) for application to the BM was used. The laser device was equipped with a metal-backed glass fiber optic (1.5 mm diameter). An infrared viewer (Laso- tronic Inc. Zug, Switzerland) and infrared-sensitive de-tecting card (Newport, Inc., Irvine, CA) were used to de- termine the infrared irradiation area. Laser application was done by a 10 mm longitudinal cut in the skin above the medial aspect, and further delicate cleaning of the bone surface was carried out. The tip of the fiber optic (1.5 mm diameter) was placed perpendicularly to the center of the exposed medial aspect of the tibia and power den- sity of 10 mW/cm2 was applied to the BM. The laser was applied for a duration of 100 sec (energy density 1.0 J/cm2). Left or right exposed tibias were chosen at random for LLLT application. In sham-operated infarcted rats that served as control the tibias were exposed and the fi- ber optic was placed as described above but the laser beam was not turned on.
2.3. Histology and Electron Microscopy
A defined cross-section sample (2 mm thick) from the central part of the infarcted area was taken from all hearts for histology. Eight micron paraffin sections were pre- pared from the tissue samples of each heart. Infarct size was determined using Masson’s trichrome staining as described by us previously [23]. Three observers, blinded to control or laser-treated rats, analyzed infarct size. Six microscopic slides from the infarcted area of each heart were chosen at random for determination of infarct size. Infarct size was expressed as the percentage of the total infarcted area relative to the total area of the left ventri- cle (LV) in each section, using image analysis software Sigma Scan Pro (Sigma, St. Louis, MO).
For electron microscopy three tissue samples from each of the control and laser-irradiated rat hearts were taken from the interphase zone between the infarcted and non-infarcted tissue by macroscopic examination. Fixa- tion was performed in 3.5% glutaraldehyde in 0.1 M ca- codylate buffer for 24 hrs followed by embedment in Epon-812. Semi-thin sections (1 micron) were prepared in order to localize the interphase zone. Thin sections were then prepared and stained with uranyl acetate and lead citrate followed by examination with a Jeol electron microscope.
2.4. Immunohistochemistry
The total number of cells immunostained for desmin (bone marrow cells or newly formed) in the infarcted area were determined using a desmin kit (Zytomed Laboratory, Ber- lin, Germany). The procedure was performed at room temperature with anti-mouse (dilution 1:25 - 1:50) primary antibody for 60 min. Following washing, slides were in- cubated with HRP secondary antibody for mouse for 30 min followed by DAB Chromogen system (Covance Inc., Dedham). Slides were rinsed again in wash buffer, stain- ed in Hematoxylin for nuclei detection, mounted and viewed using a Zeiss microscope equipped with a camera and video screen. The total number of desmin immuno- stained cells within the infarcted area was counted and their density expressed as the percentage of the total area of the infarct using SigmaPro software.
2.5. Flow Cytometry Analysis
Blood samples were taken 2 and 7 days post-IR injury for fluorescence-activated cell sorting (FACS) analysis. 100 μl of blood were mixed with different antibodies: anti-mouse CD117 (c-kit) PE (eBioscience San Diego, USA) and rat IgG2b isotype control PE (eBioscience San Diego, USA) and anti-rat macrophage marker PE (eBio- science San Diego, USA) and mouse IgG2a K isotype control PE (eBioscience San Diego, USA), were used for the FACS analysis according to the manufacturer’s guide- lines. Forty five min post incubation of the whole fresh blood with the relevant antibodies, 2 ml of Fix/Lyse so- lution (eBioscience, San Diego, USA) was added. After mixture the suspended cells were left for 60 min in the dark at room temperature. Centrifugation was performed for 10 min, supernatant was removed and washing of the pellet was performed with 2 ml of Flow Cytometry Stain- ing Buffer Solution (eBioscience, San Diego, California, USA). After another centrifugation for 10 minutes the supernatant was decanted. The pellet containing mono- nucleated cells was resuspended in 200 μl of flow stain buffer for FACS analysis.
2.6. Statistical Analysis
The SigmaStat 2.0 (Sigma, St. Luis, USA) software was used for statistical analysis. Tests were performed first for normality distribution, followed by parametric (stu- dent’s t-test) test.
3. RESULTS
Application of LLLT to the infarcted heart caused a sig- nificant (p = 0.049) reduction of 55% in infarct size as compared to control. The present of macrophages and c- kit positive cells in the blood was determined by FACS analysis (Figure 1). It was found that at 5 days post MI there was a statistical significant 2-fold higher concentra- tion of macrophages and significant 1.4-fold higher c-kit positive cells (mesenchymal cells) in the laser treated rats as compared to the infarcted non laser treated rats. Des- min immunostaining of histological sections of the in- farcted zone from laser-treated rats demonstrated a higher density of positively stained cells than in the non laser-treated ones (Figures 2-4). In the interphase zone, cells extending from the myocardium towards the in 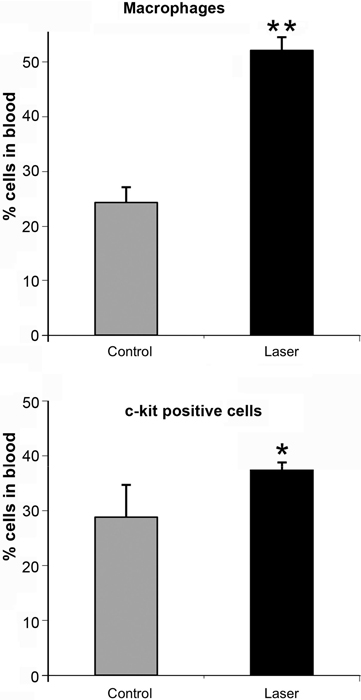
Figure 1. Percent (out of total mononucleated cells) of macro- phages and c-kit positive cells in blood of control and laser treated rats (to the bone marrow) 5 days post MI as revealed by FACS analysis. The results are mean ± S.E.M of 15 rats at each group. Statistical significance *p < 0.05; **p < 0.01.
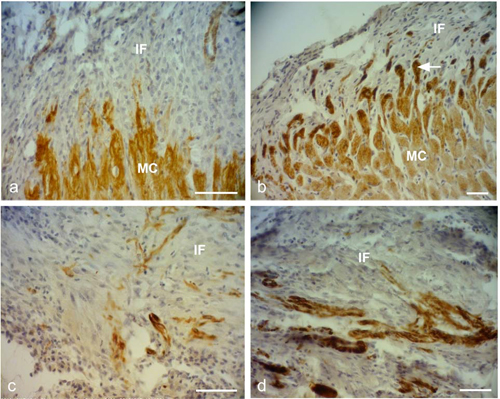
Figure 2. Representative desmin immunostained light micro- graphs of the infarcted zone of non-laser-treated rats (a, c) and laser-treated rats (to the bone marrow at 4 and 48 hrs and 5 days) (b, d) taken 2 weeks post-MI. Note that the zone in the control non-laser-treated rats contains mainly collageneous mate- rial with a few desmin immunopositive cells in the infarcted area (a, c); while in the laser-treated rats the zone displays posi- tive desmin staining in extended outgrowths (arrow) from the myocardium (MC) in (b), and in the cytoplasm of many cells in the infarcted area in (d). IF, Infarcted area. Bar = 50 μm.
farcted area showed higher immunostaining for desmin in the laser-treated rat hearts as compared to the control non-treated ones (Figure 2). The cell density of desmin immune-positive cells was also determined quantitatively in histological sections of both the infarcted laser-treated rats and infarcted non-laser-treated rats. The cell density was significantly (p < 0.01) 3-fold higher in the infarcted area of the laser-treated rats as compared to the non-la- ser-treated controls (Figure 4).
The electron micrographs of all samples taken from the control non-laser-treated infarcted rat hearts revealed a typical interphase area between intact and infarcted heart (Figure 5(a)). Adjacent to the non-ischemic intact myocardium there were conspicuous fibroblasts with col- lagen deposition dispersed among them (Figure 5(a)). In all samples taken from the laser-irradiated hearts the in- terphase zone between intact and infarcted area demon- strated different characteristics to those of the non-laser- treated infarcted rat hearts. Cells with newly-formed or- ganized contractile myofilaments dispersed in the cyto- plasm were detected in groups of several cells (Figure 5(b)). In these cells numerous mitochondria, clusters of ribosomes, and conspicuous clusters of contractile pro- teins were evident in the cytoplasm (Figures 6-8). Some cells contained dispersed contractile myofilaments in the cytoplasm that were still in an early stage of organization (Figure 6). The organization of newly-formed contractile myofilaments in the cytoplasm was observed in various
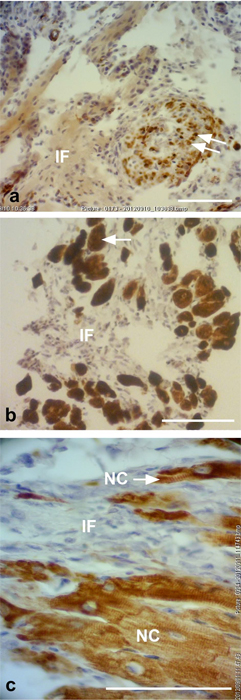
Figure 3. Representative desmin immunostained light micro- graphs of the interphase of the infarcted zone of laser-treated rats. Note that desmin positively stained cross-sections of myo- fibers (arrows) intermingled in the infarcted zone in (a). In (b) immunopositively stained cross-sections of myofibers (arrow) are visible in the infarcted area (IF). In (c) newly-formed car- diomyocytes (NC) are seen, with the desmin immunostaining mainly confined to the Z-line. Bar = 50 μm.
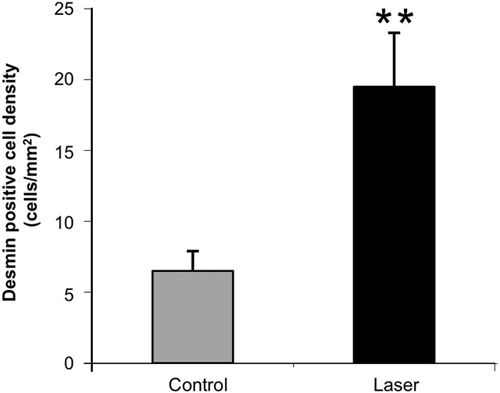
Figure 4. Density of desmin positively stained area (relative to total area) in the infarcted areas of control (non-laser-treated) and laser-treated (to the bone marrow) rats at 14 days post-MI. Results are mean+ S.E.M from 6 - 8 rats in each group. **p < 0.01.
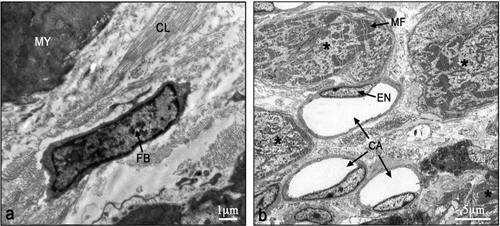
Figure 5. Electron micrographs of typical interphase zone be- tween myocardium and infarcted area of control non-laser- treated (a) and laser-treated (b) to bone marrow rats. Note intact myocardium (MY) and adjacent fibroblast (FB) in the infarcted area surrounded by collagen (CL) deposition in (a). In (b) sev- eral newly-formed cardiomyocytes (marked with asterix) with conspicuous well-organized myofilaments (MF) in their cyto- plasm are evident adjacent to blood capillaries (CA). EN, En- dothelial cell.
degrees of maturation in those cells. In some cells the myofilaments were dispersed in the cytoplasm and in others they were organized in clusters anchored to well- developed Z-lines (Figure 7(a)). In certain cells the myo- filaments were organized parallel to the longitudinal di- rection of the cells, resembling the morphological char- acteristics of mature intact cardiomyocytes (Figure 7(b)). Some of the cells were also seen in a process of forma- tion of typical intercalated disc between them (Figure 9).
4. DISCUSSION AND CONCLUSION
The most significant outcome of this study was the ap- pearance of newly-formed cardiomyocytes following laser treatment to the BM, as indicated by light and electron microscopy. There was a 3-fold increase in the density of
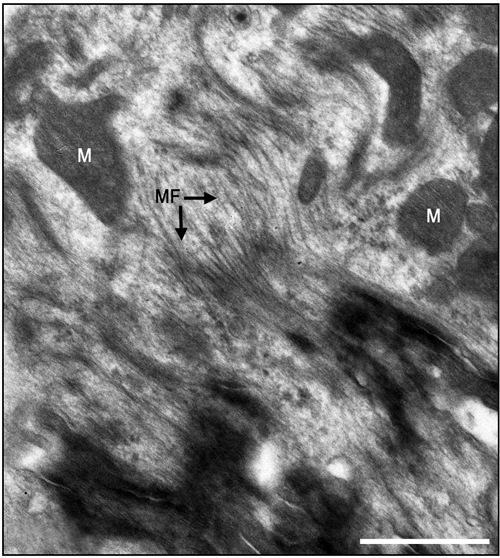
Figure 6. Electron micrographs of most probably newly-formed cardiomyocytes at an early stage of organization of contractile myofilaments. Note myofilaments (MF) in the cytoplasm. M, Mitochondrion. Bar = 1 μm.
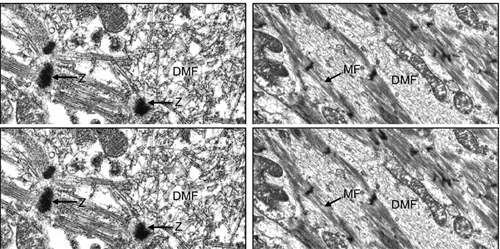
Figure 7. Electron micrographs of most probably newly-formed cardiomyocytes with early (a) and late (b) stages of the organi- zation of the contractile myofilaments in the cytoplasm. Note contractile myofilaments that are dispersed (DMF) in the cyto- plasm with a few organized in clusters anchored to Z-lines (Z) in (a). In (b) myofilaments (MF) are organized in parallel to the longitudinal axis of the cardiomyocyte, resembling their orga- nization in mature cardiomyocyte. N, Nucleus. Bar = 1 μm.
desmin immunostained cells in the infarcted rat hearts that had been laser treated. Desmin is a protein found in the cytoplasm of developing myocytes and cardiomyo- cytes [32]. The significantly higher occurrence of des- min-positive cells in the infarcted area of the laser- treated hearts may indicate the synthesis of new contrac- tile proteins in the developing new cardiomyocytes, re- sembling the process that takes place during embryonic development. The ultrastructural features of the cells in the interphase between the intact myocardium and the
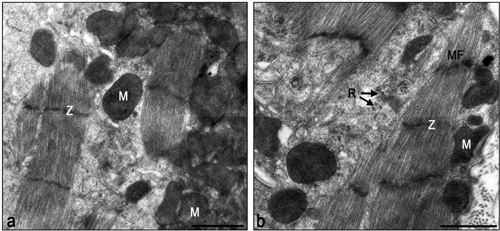
Figure 8. Electron micrographs of typical interphase zone be- tween myocardium and infarcted area of laser-treated infarcted rat heart. Note numerous mitochondria (M) in the cytoplasm of the cardiomyocytes in (a) and (b). Also note organized contrac- tile myofilament with well-developed Z-lines (Z), some dis- persed myofilaments and clusters of ribosomes (R). Bar = 1 μm.
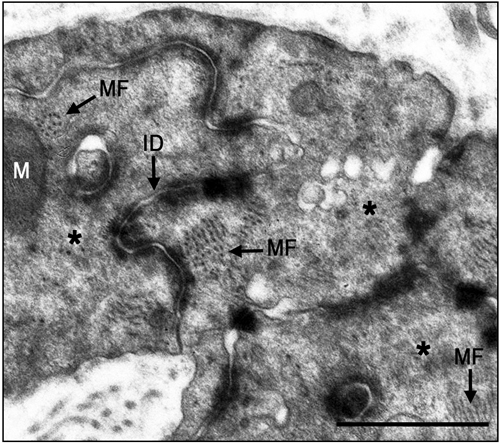
Figure 9. Electron micrographs of typical intercalated disk formation in the interphase region of the infarcted heart of la- ser-treated rats. Formation of intercalated disks (ID) between cells (marked with asterix) is evident. Note that the most proba- bly newly-formed cardiomyocytes contain clusters of myofila- ments (MF) in the cytoplasm that are conspicuous in their obli- que or cross-sections (arrows). Bar = 1 μm.
infarcted myocardium of the laser-treated rats, as shown in this study, clearly resemble the characteristics of car- diomyocytes during embryonic development of the heart [33]. Furthermore, the clusters of ribosomes and the nu- merous clusters of mitochondria in the cytoplasm of these cells may characterize cells that are active in the synthe- sis of proteins. It was previously demonstrated that direct LLLT to the infarcted hearts of rats, dogs and pigs caus- ed a significant reduction of scarring post-MI [23,24]. It was suggested that part of this reduction could be ex- plained by the regenerative response that takes place in the interphase zone [24].
The results of the present study indicate that the LLLT
applied to autologous BM attenuates the concentration of macrophages and MSC in the circulating blood. We have previously shown that LLLT application to the BM of infarcted rats caused a 2 fold enhancement in the rate of proliferation of MSC in the BM [30]. Those cells that most probably leave the BM to the circulating blood in- deed show a significant elevation of their concentration (as reveled by the FACS analysis in the present paper) at 5 days post MI. Consequently these cells probably home in on the infarcted heart, and even migrate specifically to the infarcted area [30]. These cells may induce cardiac stem cells to differentiate to newly-formed cardiomyo- cytes, as suggested previously by Hatzistergos et al. [14]. Indeed, it was found that endogenous c-kit+ cardiac stem cells were increased by 20-fold in the rat infarcted heart compared to control, following transcardial injection of BM-derived MSCs [14]. Such induction may be enabled due to paracrine secretion of various growth factors by the laser-stimulated MSC that originated from the BM. The possibility that paracrine secretion occurs in im- planted stem cells during cell therapy to the heart post- MI has been suggested previously [34]. Another mecha- nism that may take place after homing of stem cells to the infarcted heart of the laser-stimulated rats is that these cells continue to proliferate in the appropriate mi-lieu of the interphase zone in the infarcted heart and then differentiate to cardiomyocytes [30].
Another possible mechanism that maybe associates with the reduction of infarct size is the significant increase in the concentration of macrophages in the circulation fol- lowing LLLT to the BM as revealed from the FACS analysis in the present study. These findings corroborate with studies indicating that macrophages activity in the infarcted area at early stages post MI cause reduction of scarring post MI [35,36]. Thus, it could be postulated that more macrophages that will eventually home in the infarcted area from the circulating blood in the laser treated rats will also contribute to the reduction of scar- ring.
Although the findings of the present study do not in- dicate the extent of regenerative capacity of the rat in- farcted heart post-laser-irradiation, they do reveal a shift from practically no cardiomyocytes in the tissue samples taken from the non-laser-treated hearts, to the presence of newly-formed cardiomyocytes in all the electron mi- croscope sections taken from the hearts of rats that are laser-treated to the BM.
In conclusion, to the best of our knowledge, this is the first study to demonstrate the appearance of newly-form- ed cardiomyocytes in the infarcted area following LLLT to autologous BM in the infarcted rat heart. The mecha- nisms associated with this phenomenon remain to be elu- cidated in further studies.
5. ACKNOWLEDGEMENTS
This study was partially supported by the Elizabeth and Nicholas Shle- zak Super-center for Cardiac Research and Medical Engineering. The authors wish to acknowledge N. Paz for editing the manuscript and V. Wexler for helping with preparation of the figures.
REFERENCES
[1]
Rumyantsev, P.P. (1977) Interrelations of the prolifera- tion and differentiation processes during cardiac myoge- nesis and regeneration. International Review of Cytology, 51, 186-273. doi:10.1016/S0074-7696(08)60228-4
[2]
Poss, K.D., Wilson, L.G. and Keating, M.T. (2002) Heart regeneration in zebrafish. Science, 298, 2188-2190. doi:10.1126/science.1077857
[3]
Rumyantsev, P.P. (1973) Post-injury DNA synthesis, mi- tosis and ultrastructural reorganization of adult frog car- diac myocytes. An electron microscopic-autoradiographic study. Z Zellforsch Mikrosk Anat, 139, 431-50. doi:10.1007/BF00306596
[4]
Barnett, P. and van den Hoff, M.J.B. (2011) Cardiac re- generation: Different cells same goal. Medical & Biologi- cal Engineering & Computing, 49, 723-732. doi:10.1007/s11517-011-0776-5
[5]
Bollini, S., Smart. N. and Riley, P.R. (2011) Resident car- diac progenitor cells: At the heart of regeneration. Jour- nal of Molecular and Cellular Cardiology, 50, 296-303. doi:10.1016/j.yjmcc.2010.07.006
[6]
Choi, W.Y. and Poss, K.D. (2012) Cardiac regeneration. Current Topics in Developmental Biology, 100, 319-343. doi:10.1016/B978-0-12-387786-4.00010-5
[7]
Laflamme, M.A. and Murry, C.E. (2011) Heart regenera- tion. Nature, 473, 326-335. doi:10.1038/nature10147
[8]
Steinhauser, M.L. and Lee, R.T. (2011) Regeneration of the heart. EMBO Molecular Medicine, 3, 701-712. doi:10.1002/emmm.201100175
[9]
Urbanek, K., Torella, D., Sheikh, F., De Angelis, A., Nur- zynska, D., Silvestri, F., Beltrami, C.A., Bussani, R., Bel- trami, A.P., Quaini, F., Bolli, R., Leri, A., Kajstura. J. and Anversa, P. (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proceedings of the National Academy of Sciences of the USA, 102, 8692-8697. doi:10.1073/pnas.0500169102
[10]
Bittner, R.E., Schofer, C., Weipoltshammer, K., Ivanova, S., Streubel, B., Hauser, E., Freilinger, M., Höger, H., Elbe- Bürger, A. and Wachtler, F. (1999) Recruitment of bone- marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anatomy and Embryology, 199, 391-396. doi:10.1007/s004290050237
[11]
Jackson, K.A., Majka, S.M., Wand, H., Pocius, J., Hart- ley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation, 107, 1395-1402. doi:10.1172/JCI12150
[12]
Pfister, O., Mouquet, F., Jain, M., Summer, R., Helmes, M., Fine, A., Colucci, W.S. and Liao, R. (2005) CD3- but not CD31+ cardiac side population cells exhibit functio- nal cardiomyogenic differentiation. Circulation Research, 97, 52-61. doi:10.1161/01.RES.0000173297.53793.fa
[13]
Balsam, L.B., Wagers, A.J., Christensen, J.L., Kofidis, T., Weissman, I.L. and Robbins, R.C. (2004) Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature, 428, 668-673. doi:10.1038/nature02460
[14]
Hatzistergos, K.E., Quevedo, H., Oskouei. B.N., Hu, Q., Feigenbaum, G.S., Margitich, I.S., Mazhari, R., Boyle, A.J., Zambrano, J.P., Rodriguez, J.E., Dulce, R., Pattany, P.M., Valdes, D., Revilla, C., Heldman, A.W., McNiece, I. and Hare, J.M. (2010) Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differ- entiation. Circulation Research, 107, 913-922. doi:10.1161/CIRCRESAHA.110.222703
[15]
Porrello, E.R., Mahmoud, A.I., Simpson, E., Hill, J.A., Richardson, J.A., Olson, E.N. and Sadek, H.A. (2011) Transient regenerative potential of the neonatal mouse heart. Science, 331, 1078-1080. doi:10.1126/science.1200708
[16]
Conlan, M.J., Rapley, J.W. and Cobb, C.M. (1996) Bio- stimulation of wound healing by low energy laser irradia- tion. Journal of Clinical Periodontology, 23, 492-496. doi:10.1111/j.1600-051X.1996.tb00580.x
[17]
Karu, T. (2007) Ten lectures on basic science of laser photherapy. Prima Books, Gragesberg.
[18]
Karu, T. (2010) Mitochondrial mechanisms of photobio- modulation in context of new data about multiple roles of ATP. Photomedicine and Laser Surgery, 28, 159-160. doi:10.1089/pho.2010.2789
[19]
Bibikova, A. and Oron, U. (1993) Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius mu- scle by low energy laser irradiation. Anatomical Record, 235, 374-380. doi:10.1002/ar.1092350306
[20]
Bibikova, A., Belkin, A. and Oron, U. (1994) Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low energy laser irradiation. Anatomy and Embryology, 190, 597-602. doi:10.1007/BF00190110
[21]
Oron, U. (2006) Photoengineering of tissue repair in ske- letal and cardiac muscles. Photomedicine and Laser Sur- gery, 24, 111-120. doi:10.1089/pho.2006.24.111
[22]
Yaakobi, T., Shoshani, Y., Levkovitz, S., Rubin, O., Ben- Haim, S.A. and Oron, U. (2001) Long term effect of low energy laser irradiation on infarction and reperfusion in- jury in the rat heart. Journal of Applied Physiology, 90, 2411-2441.
[23]
Oron, U., Yaakobi, T., Oron, A., Hayam, G., Gepstein, L., Wolf, T., Rubin, O. and Ben Haim, S.A. (2001a) Attenu- ation of the formation of scar tissue in rats and dogs post myocardial infarction by low energy laser irradiation. La- sers in Surgery and Medicine, 28, 204-211. doi:10.1002/lsm.1039
[24]
Oron, U., Yaakobi, T., Oron, A., Mordechovitz, D., Shof- ti, R., Hayam, G., Dror, U., Gepstein, L., Wolf, T., Hau- denschild, C. and Ben Haim, S.A. (2001b) Low energy la- ser irradiation reduces formation of scar tissue following myocardial infarction in dogs. Circulation, 103, 296-301.doi:10.1161/01.CIR.103.2.296
[25]
Tuby, H., Maltz, L. and Oron, U. (2006) Modulations of VEGF and iNOS in the rat heart by low energy laser irra- diation are associated with cardioprotection and enhanced angiogenesis. Lasers in Surgery and Medicine, 38, 682- 688. doi:10.1002/lsm.20377
[26]
Oron, U., Ilic, S., De Taboada, L. and Streeter, J. (2007) Ga-As (808 nm) laser irradiation enhance ATP produc- tion in human neuronal cells in culture. Photomedicine and Laser Surgery, 25, 180-182. doi:10.1089/pho.2007.2064
[27]
Tuby, H., Maltz, L. and Oron, U. (2007) Low-level laser irradiation (LLLI) promotes proliferation of mesenchy- mal and cardiac stem cells in culture. Lasers in Surgery and Medicine, 39, 373-378. doi:10.1002/lsm.20492
[28]
Li, W.T., Leu, Y.C. and Wu, J.L. (2010) Red-light light- emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchy- mal stem cells. Photomedicine and Laser Surgery, 28, S-157-S-165. doi:10.1089/pho.2009.2540
[29]
Mvula, B., Moore, T.J. and Abrahamse, H. (2010) Effect of low-level laser irradiation and epidermal growth factor on adult human adipose-derived stem cells. Lasers in Medical Science, 25, 33-39. doi:10.1007/s10103-008-0636-1
[30]
Tuby, H., Maltz, L. and Oron, U. (2011) Induction of au- tologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers in Surgery and Medicine, 43, 401-409. doi:10.1002/lsm.21063
[31]
Oron, U. (2011) Light therapy and stem cells: A thera- peutic intervention of the future. Journal of Interventio- nal Cardiology, 3, 627-629.
[32]
Toma, C., Pittenger, M.F., Cahill, K.S., Byrne, B.J. and Kessler, P.D. (2002) Human mesenchymal stem cells dif- ferentiate to a cardiomyocyte phenotype in the adult mu- rine heart. Circulation, 105, 93-98. doi:10.1161/hc0102.101442
[33]
Oron, U. and Mandelberg, M. (1985) Focal regeneration in the rat myocardium following cold injury. Cell Tissue Research, 241, 459-463. doi:10.1007/BF00217194
[34]
Mummery, C.L., Davis, R.P. and Krieger, J.E. (2010) Challenges in using stem cells for cardiac repair. Science Translational Medicine, 14, 1-5.
[35]
van Amerongen, M.J., Harmsen, M.C., van Rooijen, N., Petersen, A.H. and van Luyn, M.J. (2007) Macrophage dep- letion impairs wound healing and increases left ventricu- lar remodeling after myocardial injury in mice. American Journal of Pathology, 170, 1093-1103. doi:10.2353/ajpath.2007.060547
[36]
Okazaki, T., Ebihara, S., Asada, M., Yamanda, S., Saijo, Y., Shiraishi, Y., Ebihara, T., Niu, K., Mei, H., Arai, H. and Yambe, T. (2007) Macrophage colony-stimulating factor improves cardiac function after ischemic injury by induc- ing vascular endothelial growth factor production and sur- vival of cardiomyocytes. American Journal of Pathology, 171, 1093-1103. doi:10.2353/ajpath.2007.061191
Original Source: http://www.scirp.org/journal/jbise
Influence of Low Level Laser Radiation on Migration of Stem Cells
Levon Gasparyan, Grigory Brill, Anu Makela - (Publication) 4468
This study showed a 26% increase in stem cell when they uses red and IR lasers continuous wave.
View Resource
Abstract: The long term effects of low level laser therapy can involve treatment mechanisms connected with activation of stem cells.
In the current study migration of stem cells was tested under the influence of laser light alone as well as in case of combined influence of light and stromal cell-derived factor-1α (SDF-1α). This cytokine plays a role in lymphocyte trafficking, hematopoietic progenitor cell and stem cell homing.
To investigate the light influence on stem cells, we analyzed factor-dependent cell-Patersen (FDCP)-mix multipotent progenitor cells.
Migration of the stem cell line was tested using Transwell system (Corning, NY) under influence of red diode laser (λ=659.6 nm, 19.5 mW) or infrared diode laser (λ=958 nm, 36 mW) during 15 min at continuous wave, as well as in case of applying 150 ng/ml SDF-1α.
Group 1 cells were a group of control, group 2 cells received only red light irradiation, while group 3 cells had IR light irradiation. Group 4 cells were treated with 150 ng/ml SDF-1α. Group 5 cells were irradiated with red laser light in addition to 150 ng/ml SDF-1α, and group 6 cells by IR light and 150 ng/ml SDF-1α.
The count of migrated cells was 1496,5±409 (100%) in case of control. Red and IR laser light increased migration activity of stem cells up to 1892±283 (126%) and 2255,5±510 (151%) accordingly. Influence of SDF-1α was more significant, than effects of light irradiation alone 3365,5±489 (225%). Combined effects of light irradiation and SDF-1α were significantly stronger 5813±1199 (388%) for SDF-1α and red laser light, and 6391,5±540 (427%) for SDF-1α and IR laser light irradiation.
Preliminary study results showed that laser light irradiation can activate stem cell migration in vitro. The results are more reliable in the case of combined application of light and SDF-1α. These results are giving ground to consider that stem cell reactions to light irradiation can be one of the factors of light therapy.
Key words: low level laser irradiation, low level laser therapy, stem cells, SDF-1, stromal cell-derived factor-1
INTRODUCTION
More than 30 years ago first reports about biological effects of low doses of laser light were presented. Currently low level laser therapy (LLLT) is successfully applied in the treatment of numerous diseases and pathological conditions. LLLT exhibits positive effects for the treatment of disorders, having in common failure of blood supply with development of acute or chronic tissue hypoxia, different level of destruction of tissues, following decreased regenerative abilities of tissues and organs, defects in immune system, and altered cell metabolism. At the same time some important mechanisms of influence of laser light on the body are still far to be fully understood [1 - 8].
Recent studies discovered important role of bone marrow hematopoetic stem cell (HSCs) for naturally occurred recovery and regeneration processes, following tissue hypoxia and injury. The three clinically important steps in this natural process are mobilization of stem cells from the bone marrow, homing of these cells to the site of injury, and differentiation of the stem cell into a functional cell of the injured tissue [9]. Different methods of stem cell therapy, the treatment method, based on mobilization and transplantation of stem cells, proves to be effective method of therapy for different disorders.
We proposed a hypothesis that wide range of positive effects following laser therapy can be connected to increased activity of stem cells in damaged tissues. To test that, we examined in vitro the influence of laser light on migration of stem cells in absence and in presence of stromal cell-derived factor-1 (SDF-1), a potent chemoattractor for lymphocytes, monocytes, HSCs, which plays a critical role in the stem cell migration towards areas of tissue injury and hypoxia.
MATERIALS AND METHODS
To investigate the light influence on stem cells, we analyzed factor-dependent cell-Patersen (FDCP)-mix multipotent progenitor cells. The FDCP-mix stem cell line was maintained in ISCOVE’S medium supplemented with 20% horse serum and penicillin/streptomycin in the presence of 20 ng/ml IL-3. The cells were supplied with fresh medium each 5 days. Migration of the stem cell line was tested using Transwell system (Corning, NY). The cells were washed with PBS once and re-suspended in the medium containing 0.1% BSA (2x106/ml). Then, 600 μl of the mixture was irradiated by red diode laser (λ=659.6 nm, 19.5 mW) or infrared diode laser (λ=958 nm, 36 mW) during 15 min at continuous wave. Next, 100 μl of the mixture (2x105 cells) was seeded into upper chambers of the Transwell system, and the filters were placed into the wells containing 600? μl of the medium with or without 150 ng/ml SDF-1α. The plate was incubated for 4 h (37°C, 5% CO2, humidified atmosphere), after which the cells were collected and counted by a FACS sorter (Beckton Dickinson) during 1 min. All samples were performed in duplicate.
Group 1 cells are control group, group 2 cells received only red light irradiation, while group 3 cells – only IR light irradiation. Group 4 cells were treated with 150 ng/ml SDF-1α. Group 5 cells were irradiated with red laser light in addition to 150 ng/ml SDF-1α, and group 6 cells – IR light and 150 ng/ml SDF-1α.
RESULTS
Small amount of stem cells can migrate without SDF-1α or laser light influence. The count of migrated cells in control group was 1496,5±409 (Fig). This amount was considered as 100%. Red and IR laser light at the above mentioned dosage and methods of irradiation increased migration activity of stem cells up to 1892±283 (126%) and 2255,5±510 (151%) accordingly. Influence of SDF-1α was more noticeable, than effects of red or IR laser light irradiation alone - 3365,5±489 (225%). It is important to stress attention on the finding, that rate of stem cell migration towards the filter and SDF-1α containing medium was much higher after laser irradiation of cells - 5813±1199 (388%) for red laser light, and 6391,5±540 (427%) for IR laser light irradiation.
DISCUSSION
The main scientific result of this study is the fact, that red and infrared laser light irradiation can activate migration of stem cells in vitro. Moreover, red and IR laser radiation can up-regulate the rate of stem cell migration towards higher SDF-1α gradient.
How to explain the direct effects of mobility of stem cells in vitro under red and IR laser light irradiation, and use this fact for better understanding the wide range of therapeutic effects of laser therapy?
Modern medical science has accepted that every pathologic condition or disease should be treated according to its clinical stage and symptoms, considering its pathogenesis and etiology. Similar treatment methods can be applied only for the treatment of different diseases, having common pathogenesis.
Not very many examples of successful application of the similar or close therapy method for the treatment of different pathologies are known in modern medicine. Steroid hormone therapy is one of such cases.
Another illustration of successful application of the similar treatment techniques for treatment of different disorders is stem cell therapy, a novel treatment method, which is still under development. Growing data suggests, that transplanted stem cell can successfully and for long period of time improve heart myocardial contractility and other heart functions after myocardial infarction, can support neoangiogenesis in areas of tissue infarction and damage, can replace several cell types in tissues, including β-cells in diabetes models, neurons, cardiomyocytes, hematopoetic cells of different lineages and so on, as well as be useful in the treatment of atherosclerosis [9].
The main principle of stem cell therapy is the idea of replacement of damaged and dead cells in injured tissues and organs with new healthy ones. It is known, that severe stress, tissue hypoxia and damage mobilizes some hematopoetic stem cells (HSCs) from bone marrow to peripheral bloodstream. After that HSCs can migrate towards hypoxic tissues and reach them. Finally they can start to proliferate to the cells types, typical for that damaged tissues. HSCs in the tissues are also able to produce several cytokines, chemokines, growthfactors, improve survival of damaged cells and limit apoptosis. As a result of some tissue regeneration, improvement in the function of a damaged organ can be achieved. Similar and even stronger regeneration and treatment effects can be displayed after transplantation of fetal or adult HSCs to recipient [10-12].
Low laser light irradiation is one other example of application of the same factor for the treatment of number of disorders, which, at first glance, have nothing or very little in common in their pathogenesis. Laser light can accelerate wound and burn healing, improve condition of patients after myocardial infarction and stroke, can support hematopoiesis of bone marrow after X-ray radiation or during cancer chemotherapy, can help for the treatment of diabetic angiopathy and neuropathy, as well as reduce atherosclerotic plaque formation. In cellular and tissue level LLLT exhibits positive effects for the treatment of disorders, having in common failure of blood supply with development of acute or chronic tissue hypoxia, different level of destruction of tissues, following with decreased regenerative abilities of cells, as well as altered cell metabolism [6, 7, 13, 14].
One can see that the therapeutic applications of LLLT and stem cell therapy are very close. So, earlier we proposed the hypotheses that one of the mechanisms of light therapy includes acceleration of tissue repair due to better mobilization of stem cells to the spot of injury after laser light irradiation [15]. That process should include several phases, including activation of stem cell migration towards area of tissue damage and hypoxia.
Stem cells are being investigated for their potential use in regenerative medicine. Stem cells share the following two defining characteristics: the capacity to differentiate into a spectrum of different cell types and the capacity to renew themselves [16]. The biological principle that underlies stem cell therapy is tissue-directed differentiation. For example, adult stem cells isolated from liver tissue and re-injected into liver become hepatocytes, whereas the same cells injected into myocardium become myocytes. [17] Stem cells have been engrafted into a broad spectrum of tissues, including regenerating bone, neural tissue, dystrophic skeletal muscle, and injured skeletal muscle. [18]. Myocardial regeneration is perhaps the most widely studied and debated example of stem cell plasticity. The most promising results have been obtained after transplantation and mobilization of bone marrow cells to the area of infarction.
The three clinically important steps in this natural process are mobilization of stem cells from the bone marrow, homing of these cells to the site of injury, and differentiation of the stem cell into a functional cell of the injured tissue [19].
Stem cell repair of cardiac and vascular tissue is a naturally occurring process after injury [20, 21] Circulating CD34+ mononuclear cell counts and plasma levels of endothelial growth factor are significantly increased in patients with acute myocardial infarction, peaking on day 7 after onset [22]. Due to limitations of the naturally occurring repair process after myocardium infarction and other injuries or pathologies several stem cell transplantation strategies were proposed and tested.
At present, however, enthusiasm for the therapeutic potential of strategies of stem cell transplantation is limited by certain practical considerations. For example, the number of stem cells, required for injection for the treatment of myocardial infarction, can be harvested approximately from 6 l of donor blood [23].
Other important limitation for autologous bone marrow stem/progenitor cell mobilization is a recent finding, that circulating endothelial progenitor cells in patients with coronary heart disease are impaired with respect to number and functional activity. Moreover, Heeschen et al [24] reported that regeneration and functional ability of bone marrow-derived mononuclear cells (BM-MNCs) in patients with chronic ischemic cardiomyopathy (ICMP) are also limited. In spite of the fact that, the number of BM-MNCs isolated from bone marrow aspirates of 18 patients with ICMP and 8 healthy subjects s did not differ, the colony-forming capacity of BM-MNCs from patients with ICMP was significantly lower compared with BM-MNCs from healthy controls. Likewise, the migratory response to SDF-1 and vascular endothelial growth factor (VEGF) was significantly reduced in BM-MNCs derived from patients with ICMP compared with BM-MNCs from healthy controls. The reduced neovascularization capacity in vivo of BM-MNCs derived from patients with ICMP closely correlated with the in vitro assessment of SDF-1-induced migration and colony-forming capacity.
The need for development of new methods for mobilization, as well as for homing of stem cells to the site of injury is therefore evident.
Several growth factors, chemokines and cytokines are involved in the regulation of stem cell mobilization, homing and differentiation. Stromal cell-derived factor-1 (SDF-1) is one of them. SDF-1 is a chemokine playing an important role in the trafficking of hematopoietic stem cells. SDF-1 is expressed on stromal cells of various tissues. CXCR4 is the only known receptor for SDF-1 [25]. SDF-1/CXCR4 interaction is reported to play an important physiological role during embryogenesis in hematopoiesis, vascular development, cardiogenesis, and cerebellar development [26-28].
Recently, several investigators have reported that CD34+ cells, classically considered to be hematopoietic stem cells, expressed CXCR4, and that SDF-1 could induce CD34+ cell migration in vitro [29]. Accordingly, SDF-1 is considered as one of the key regulators of hematopoietic stem cell trafficking between the peripheral circulation and bone marrow. SDF-1 has also been shown to effect CD34+ cell proliferation and mobilization and to induce angiogenesis in vivo [30 -32].
Hattori et al [31] reported that plasma elevation of SDF-1 induced mobilization of mature and immature hematopoietic progenitors and stem cells, including endothelial progenitor cells (EPCs). However, application of granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization is widely accepted nowadays.
Yamaguchi et al [23] studied the effects of SDF-1 on migration and accumulation of EPCs. SDF-1 induced EPCs migration in a dose dependent manner in vitro. The magnitude of migration was similar to that induced by VEGF. Authors also reported that locally (in hind-limb ischemic muscle of experimental animals) administered SDF-1 could augment the local accumulation of transplanted EPCs from peripheral blood, thereby resulting in enhanced neovascularization. As a result, cell transplantation not only improved neovascularization but also reduced adverse biological consequences such as limb necrosis and auto-amputation in the mouse ischemic hind-limb model. These studies also disclosed that systemic EPCs transplantation improved myocardial neovascularization and cardiac function corresponding to reduced left ventricular scarring. Authors concluded that, at least under the experimental conditions used in the study, the effect of SDF-1 on neovascularization appears to result primarily from its ability to enhance the recruitment and incorporation of transplanted EPCs.
Damas at al. [33] reported that SDF-1α, at least in high concentrations, may mediate anti-inflammatory and matrix-stabilizing effects in unstable angina. These effects may promote plaque stabilization, and therapeutic intervention that enhances SDF-1 α activity could potentially be beneficial in acute coronary syndromes. Authors demonstrated significantly altered SDF-1/CXCR4 expression in patients with angina, with particularly marked changes in those with unstable disease, with low SDF-1 levels in plasma and altered expression of its corresponding receptor on peripheral blood mononuclear cells (PBMC). In contrast to the raised plasma levels of inflammatory chemokines in patients with angina plasma levels of SDF-1 and the surface expression of its corresponding receptor (CXCR4) on PBMC appear to be down-regulated in these patients. Thus, although persistent inflammation may involve up-regulation of inflammatory chemokines, recent studies suggest that inflammatory cytokines (eg, TNF-α and IL-1) may decrease the expression of SDF-1 and CXCR4.
Future progress of stem therapy techniques probably will include development of incubation methods for enhancement stem cell mobility and homing ability, as well as for faster proliferation into desire tissue cells. Increasing migration abilities will help to achieve better and faster results.
The ability of laser light to activate migration and mobility of different cells is well known. It was noticed, that irradiation of sperm cells in vitro can increase their mobility and fertility [34]. Moreover, this effect is more pronounced in case of damaged cells with low mobility rate. This gives a ground to assume that laser light irradiation in certain dosage and condition can improve functional abilities of cells. Future experiments are required to ascertain if stem cells respond to the laser light the same way.
The main finding on this study is that red and IR laser light can stimulate stem cell migration in vitro, and especially increase migration towards SDF-1α gradient. Stem cell ability to migrate towards tissues with higher SDF-1 concentration is one of the key mechanisms of stem cell homing. These results are giving ground to speculate that activation of stem cell migration can be one of the mechanisms of low level laser therapy. Taking into consideration that the combined of SDF-1 and laser irradiation had the strongest effect on stem cell homing, it would be reasonable to assume that this combination could be used in not only increasing the activity of stem cells but also in determining the main area of stem cell mobilization and homing. The current study did not aim to study the mechanisms of increased migration ability, which will be study in the future. But it is possible to suggest following explanation: laser irradiation can change the metabolism of stem cells, increase ATP production and so increase the migration, as well as up-regulate CXCR4 receptor expression or syntheses de novo. More studies are required to test if the laser light irradiation in vivo is able to make homing of transplanted stem cells to the area of damage more efficient, to check the influence of laser light on the mobilization rate of stem cells from bone marrow, to investigate if laser light can enhance functional abilities of stem cells. These studies would be desirable for better understanding of the mechanisms of laser therapy and for development of more effective methods of stem cell therapy.
References
1. Tuner J. and Hode L. Low Level Laser Therapy: Clinical Practice and Scientific Background, Prima Books, Grängesberg, Sweden, 1999.
2. Karu T. The Science of Low Power Laser Therapy, Gordon & Breach, London, 1998.
3. Baxter G.D. Therapeutic Lasers: Theory and Practice, Churchill Livingstone, London, 1994.
4. Simunovic Z., Ed. Lasers in Medicine and Dentistry, Vitgraf, Rijeka (Croatia), 2000.
5. Zhukov B.N. and Lysov N.A. Laser irradiation in experimental and clinical angiology (in Russian), Samara (Russia), 1996.
6. Kozlov V.I., et al. Bases of laser physio- and reflexo-therapy (in Russian), Zdorovje, Samara (Russia), 1993.
7. Paleev N.R. Ed. Phototherapy (in Russian), Meditsina, Moscow (Russia), 2001.
8. Skobelkin O. K. Ed. Application of low-intensive lasers in clinical practice (in Russian). Moscow, 1997.
9. Forrester J, Price M, Makkar R. Stem Cell Repair of Infarcted Myocardium. An Overview for Clinicians. Circulation. 2003;108:1139–1145.
10. Orlic D., Hill J., Arai A. Stem Cells for Myocardial Regeneration Circulation Research. 2002;91:1092.
11. Hodgson D., Behfar A., Zingman L.V., Kane G.C., Perez-Terzic C., Alekseev A.E., Puceat M., and Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol, August 1, 2004; 287(2): H471 - H479.
12. Ozbaran M., Omay S. B., Nalbantgil S., Kultursay H., Kumanlioglu K., Nart D., and Pektok E. Autologous peripheral stem cell transplantation in patients with congestive heart failure due to ischemic heart disease. Eur. J. Cardiothorac. Surg., March 1, 2004; 25(3): 342 - 350.
13. Brill A.G., Shenkman B., Brill G.E. et al. Blood irradiation by He-Ne laser induces a decrease in platelet responses to physiological agonists and an increase in platelet cyclic GMP. Platelets. 2000. Vol. 11. P. 87-93.
14. Mester A. Biostimulative effect in wound healing by continuous wave 820 nm laser diode. Lasers in Med Science, abstract issue July 1988, No. 289.
15. Gasparyan L.V. Stem cells and therapeutic effect of light irradiation (in Russian). Collection of abstracts of the 10th International Conference of Quantum Medicine, Moscow, 2003, pp. 43-44.
16. Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101.
17. Malouf NN, Coleman WB, Girsham JW, et al. Adult-derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol. 2001;158:1929–1935.
18. Donovan PJ, Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97.
19. Forrester J, Price M, Makkar R. Stem Cell Repair of Infarcted Myocardium. An Overview for Clinicians. Circulation. 2003;108:1139–1145.
20. Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757.
21. Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174.
22. Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779.
23. Yamaguchi J, Kusano K, Masuo O, at al. Stromal Cell–Derived Factor-1 Effects on Ex Vivo Expanded Endothelial Progenitor Cell Recruitment for Ischemic Neovascularization. Circulation. 2003;107: 1322–1328.
24. Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615-22.
25. Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833.
26. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638.
27. Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998; 393:591–594.
28. Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599.
29. Mohle R, Bautz F, Rafii S, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530.
30. Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34+ cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768.
31. Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360.
32. Salcedo R, Wasserman K, Young HA, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal derived factor-1α. Am J Pathol. 1999;154:1125–1135.
33. Damas J, Wæhre T, Yndestad A, et al. Stromal Cell–Derived Factor-1a in Unstable Angina. Circulation. 2002;106:36-42.
34. Pyrikova S.I et al. Effect of laser exposure on human seminal fluid (in Russian). Clinical and laboratory diagnosis. 1998;5:15-16.
Photobiomodulation of the Brain
Michael R. Hamblin and Yng-Ying Huang - 2019 (Publication) 4512
This is Hamblin and Huang's best summary of PBM for treating the brain.
View Resource
Photobiomodulation (PBM) also known as low-level laser (or light) therapy has been known for over 50 years (since
1967), but it is only relatively recently that it has begun to make the transition into the mainstream. PBM describes the
use of red or near-infrared light at levels that do not produce undue heating of the tissue to produce beneficial effects
on the human body. The introduction of light-emitting diodes (LEDs) has made this approach more accessible than the
previously used laser sources, as LEDs are safer, cheaper, and can easily be used at home. Another factor that has led
to PBM becoming more widely accepted is the growing understanding of the mechanisms of action at a molecular and
cellular level. The lack of a clear mechanism of action was a deterrent to many biomedical scientists who maintained a
healthy level of skepticism.
Among the wide range of tissues, organs, diseases, and conditions that can be beneficially affected by PBM, the subject
of this book is the brain. The brain is probably the single human organ that engenders the most concern, interest,
and expenditure in the 21st century. Brain disorders that cause widespread morbidity, mortality, and loss of quality of
life can be divided into four broad categories. Traumatic brain disorders include stroke, traumatic brain injury (TBI),
global ischemia, and perinatal difficulties. Neurodegenerative diseases include Alzheimer’s disease, Parkinson’s disease,
and a range of dementias. Psychiatric disorders include major depression, anxiety, addiction, and insomnia, among
many others. Finally there are neurodevelopmental disorders (autism and ADHD) and the possibility of cognitive
enhancement in healthy individuals. Many of these brain disorders are specifically addressed in the present volume.
The book is divided into three parts. The first part covers some basic considerations, dosimetry, and devices, and
discusses the mechanisms of action at a cellular level and on the brain as a whole organ. The second part includes contributions
from researchers who have carried out studies on a variety of animal models in their investigations of brain
disorders, stroke, TBI, and Alzheimer’s and Parkinson’s diseases, to name a few. The third part concentrates on human
studies, including controlled clinical trials, pilot trials, case series, and clinical experience. Disorders treated include
TBI, stroke, Alzheimer’s and Parkinson’s diseases, depression, and others.
The book is expected to play a role in stimulating the further increase and acceptance of PBM for brain disorders,
which has really started to take off in recent years. It will also act as a resource for researchers and physicians wishing
to get a broad overview of the field and who are contemplating entering it themselves. The number of individuals considering
obtaining a home-use PBM device is also steadily increasing and this book will act as
Original Source: https://books.google.com/books/about/Photobiomodulation_in_the_Brain.html?id=P0qiDwAAQBAJ&source=kp_book_description
A Practical Handbook: Laser Acupuncture
Volkmar Kreisel and Michael Weber - (Book) 4319
View Resource
This book is like a bible for laser acupuncture. It is the most detailed book on the subject that we have been able to find. It can be a little hard to get out because the publisher is in Germany. Dr. Weber operates a large clinic in Germany where he treat a wide variety of conditions. He also does training classes for acupuncturist and is a leader in the field of laser acupuncture. In addition to having a detailed explanation of how lasers stimulate the body, her provide some great general guidelines on the use of lasers and his book includes beautifully detailed protocols. Chapters in the book include 3 major sections: High-Tech Acupuncture with Laser Light, Practical Guidelines and Treatment Concepts. Within the treatment concepts are group of protocols for Orthopedics, Neurology, Psychosomatic disorders, Throat, Nose and Ear, Internal Medicine, Dermatology, Pediatrics, Gynecology ,Dental Medicine and Ophthalmology.
Original Source: http://www.coldlasers.org/lllt-books/
Are all the negative lllt studies really negative?
Tunér-Hode - 1998 (Publication) 4385
View Resource
This is an excerpt from the book "Low Level Laser Therapy" by Tunér-Hode, chapter 13. You will find the excerpt at the link below or here. This excerpt talks about how not all negative LLLT studies can necessarily say that LLLT does not work. The main problem being that the dose or wavelength was incorrect for the attempted treatment, leaving reasearchers with less than satisfactory results, in some cases laser parameters were not even recorded. While we must take negative studies seriously, it can be seen that once the majority of them have been examined that the attempted LLLT was simply being done incorrectly. You will find the excerpt broken up thusly:
- Are all the negative lllt studies really negative?
- "I heard it through the grapewine"
- Positive from negative
- Negative from negative
- Important parameters
- A. Wavelength
- B. Dose
- C. Power density
- Typical traditional laser instruments
- Dose development
- Pitfalls
- 1. Low outputs
- 2. Inclusion criteria
- 3. Lack of proper control groups
- 4. Therapeutic technique
- 5. Systemic effects
- 6. Tissue condition
- 7. Power density
- 8. Mixed parameters
- 9. The influence of ambient light
- 10. Premature conclusions
- 11. Meta-analyses
- Confusion between groups
Original Source: http://www.laser.nu/lllt/LLLT_critic_on_critics.htm
Light and Laser Therapy: CLINICAL PROCEDURES
Curtis Turchin, MA, DC - 2011 (Book) 4326
View Resource
This book introduces you to the science of Laser Therapy, starting with the history and basic physics of laser radiation, including things like:

- Lasers vs. LED's
- Measuring Wavelength
- The Electromagnetic Spectrum
- Depth of Laser Penetration
- Types of Laser Diodes
- Classification of Diodes
- Light Energy in Joules
- Pulsing or Frequency
- Treatment Parameters
- Light Absorbed
- Laser Safety
- Contraindications of Light Therapy
- Optimal Dose
- Calculating Output
- Dose and Time for Different Physical Qualities
You will find suggested treatments, and accompanying diagrams for the syndromes listed below:
Head and Face:
- Bell's Palsy
- Migraine Headache
- Sinusitis
- Temporomandibular Joint Syndrome (TMJ)
- Tension Headache
- Trigeminal Neuralgia
- Wrinkles
Spine and Pelvis:
- Cervicall Disc Herniation
- Cervical Stenosis
- Cervical, Thoracic, Lumbar Sprain/Strain and Neuritis
- Coccydynia
- Costochondritis
- Herniated Lumbar Disc or Annular Tear
- Lumbar Stenosis
- Pubic Symphysis Sprain
- Sacroiliac Sprain or Strain
- Spinal Hypermobility Syndrome
Systemic:
- Addiction to Cigarettes or Other Substances
- Ankylosing Spondylitis
- Arthritis
- Complex Regional Pain Syndrome or Reflex Sympathetic Dystrophy
- Fibromyalgia Syndrome (FMS)
- Herpes Zoster/Shingles and Post Herpetic Neuralgia
- Post Surgical Pain
- Wounds (Slow or Non-Healing)
Upper Body:
- Acromioclavicular (AC) Sprain or Laxity
- Biceps Tendinitis
- Biceps Tendon Strain
- Carpal Tunnel Syndrome
- DeQuervain's Tendinitis
- Dislocated Finger or Thumb
- Fractured Carpal, Metacarpal, or Phalange
- Fractured Clavicle
- Fractured Distal Radius or Casted Forearm or Hand
- Frozen Shoulder
- Ganglion Cyst of the Wrist
- Olecranon Bursitis
- Radial or Ulnar Neuritis
- Rotator Cuff Strain
- Shoulder Rheumatoid and Oseoarthritis
- Subacromial Bursitis
- Tennis and Golfer's Elbow
- Thumb or Finger Sprain
- Triceps Strain
- Wrist Flexor or Extensor Tendinitis
Lower Body:
- Achilles Tendinitis and Rupture
- Adductor Strain
- Anterior (ACP) and Posterior Compartment Pain (PCP)
- Anterior and Posterior Cruciate Ligament Injury
- Calcaneal Bursitis
- Calf Strain
- Dislocated Patella
- Hallux Valgus and Rigidus
- Hamstring or Ischiogluteal Bursitis and tendinitis
- Hamstring Strain
- Hip Sprain
- Interdigital Neuritis - Metatarsalgia - Morton's Neuroma
- Knee Contusion, Housemaid's Knee, Prepatellar Bursitis
- March or Stress Fracture
- Medial and Lateral Collateral Ligament Injury
- Meniscus Sprain/Strain
- Metatarsalgia - Thinning of the Fat Pad
- Osgood Schlatter Syndrome
- Osteochrondritis Dissecans
- Patellar Teninitis and Quadriceps Insertion Strain
- Patellofemoral Syndrome
- Peripheral Neuropathy (PN)
- Piriformis Syndrome
- Plantar Fasciitis
- Posterior Knee Swelling - Baker's Cyst
- Quadriceps Strain
- Restless Leg Syndrome or Leg Cramps
- Sesamoiditis
- Shin Splints
- Sprained Ankle
- Tarsal Tunnel Syndrome
- Tensor Fascia Lata and Iliotibial Band Syndrome
- Tibial or Fiula Stress Ftacture
- Trochanteric Bursitis
Original Source: http://www.coldlasers.org/lllt-books/
Laser Therapy and Laser Puncture in Dogs and Cats
Anja Füchtenbusch and Peter Rosin - 2012 (Book) 4327
View Resource
In the book "Laser Therapy and Laser Puncture in Dogs and Cats", you will find acupuncture points and meridians for cats and dogs, with accompanying diagrams in the appendix. This book will go over using your laser therapy system, and determining treatment times and plans; as well as protective measures, contraindications, and side effects. The bulk of the book is about Finding the right treatment plan, and is broken into color coded sections thusly:
- Guidelines
- Wounds, Scars, Disturbance Fields
- Pain
- Locomotor System
- Repiratory Tract
- Gastrointestinal Tract
- Bladder, and Kidneys
- Liver
- Spleen, and Pancreas
- Vessels
- Metabolism, and Detoxification
- Skin
- Immune System
- Psyche
- Other Disorders
Original Source: http://www.coldlasers.org/lllt-books/
Quantum Acupuncture: The Next Level
Ronald Henery DC, ND, FIAMA - 2011 (Book) 4331
View Resource
This book consists mostly of diagrams and charts explaining the different points and meridians on the human body.
Five Elements and the Chakras
- five elements
- the nurturing cycle
- the control cycle
- beyond the five elements
- 24-hour horary cycle
- chakra system in meridian therapy
The Meridians
- the meridian network
- the relation between meridians and their organs
- chapter 2 overview
- heart
- small intestine
- bladder
- kidney
- pericardium
- triple heater
- gallbladder
- liver
- lung
- large intestine
- stomach
- spleen
- governor vessel
- conception vessel
Point Group Use in Diagnosis and Treatment
- tonification and sedation points
- master points
- source points
- luo points
- accumulation points
- command points
- alarm points
- bladder association points
- ifluential points
- transporting points
- the jing-well points
- the spring points
- the stream points
- the river points
- the sea points
- lower he-sea points
- meeting points
- ma dan-yang points
- vitality collapse points
- entry-exit points
- gohst points
- pulse points
- mensturation points
- pregnancy points
- musculo-tendendo points
Key Points on the Meridians
- heart meridian
- small intestine meridian
- bladder meridian
- kidney meridian
- pericardium meridian
- triple heater meridian
- gallbladder meridian
- liver meridian
- lung meridian
- large intestine meridian
- stomach meridian
- spleen meridian
- governor vessel
- conception vessel
Point Location
- heart
- small intestine
- bladder
- kidney
- pericadium
- triple heater
- gallbladder
- liver
- lung
- large intestine
- stomach
- spleen
- governor vessel
- conception vessel
Original Source: http://www.coldlasers.org/lllt-books/
Laser Phototherapy Clinical Practice and Scientific Background
Lars Hode and Jan Tunér - 2014 (Book) 4328
This book is one of the most comprehensive resources for European style laser therapy.
View Resource
This book covers an astonishing amount of information in its near thousand pages, everthing from basic laser physics to dental, and veteranary useage. Here are some of its contents:

- Basic Laser Physics
- physics
- energy
- radiation
- wavelength and frequency
- photon energy
- the elecromagnetic spectrum
- the optical reigon
- radiation risks
- can electromagnetic radiation cause cancer
- protective mechanisms
- light
- the optical spectrum
- light sources
- various sources of radiation
- natural sources of radiation
- man-made light sources
- the light emmiting diode (LED)
- flash lamps
- the laser
- laser design
- practical lasers
- the properties of laser light coherence
- interference
- laser beam characteristics
- polarisation
- output power
- continuous and pulsed lasers
- the peak power value
- average power output
- power density
- light distribution
- beam divergence
- collimation
- risk of eye injury
- decisive factors in the risk of eye injury
- the laser instrument
- properties of some laser types
- description of common surgical laser types
- the CO2 laser (carbon dioxide laser)
- carbon dioxide lasers in surgery
- carbon dioxide lasers in dental applications
- the Nd:YAG laser
- Nd:YAG lasers in surgery
- Nd:YAG lasers in dentistry
- erbium lasers in dentistry
- "strong" diode lasers in dentistry
- the KTP laser
- Q-switching
- Theraputic Lasers
- the first generation 1975-85
- the second generation 1985-95
- the third generation 1995-2005
- the fourth generation 2005 and onwards
- what is a good laser therapy instrument
- the basic instrument
- sales tricks
- high power-low power
- laser or LED
- high or low price
- penetration of light into tissue
- "a story of a young scientist"
- the wavelength
- how deep does light penetrate into tissue?
- Biostimulation
- history
- a few words on mechanisms
- photoreceptors
- what parameters to use
- laser parameters
- whitch wavelength?
- output power
- average output power
- power density
- energy density
- the dose
- treatment dose
- calculation of doses
- dose ranges
- calculation of treatment time for a desired dose
- "reay reckoner"
- dose per point
- pulsed or continuous light
- pulse repetition rate (PRP)
- patient parameters
- treatment area
- treatment intervals
- pre- or postoperative treatment
- treatment method parameters
- local treatment
- shallow problems
- deeper problems
- treating inside the body
- systemic treatments
- acccupuncture
- trigger points
- spinal processes
- dermatome
- blood irradiation
- irradiation of lymph nodes
- irradiation of ganglions
- combo treatment
- interaction with medication
- other considerations
- what about collimation?
- depth of penetration, greatest active depth
- factors that reduce penetration
- tissue compression
- how deep does the light penetrate?
- laser light irradiation through clothes
- the importance of tissue and cell condition
- the importance of ambient light
- in vitro/ in vivo
- laser therapy with high output lasers
- laser therapy with carbon dioxide lasers
- laser therapy with Nd:YAG lasers
- laser therapy with ruby lasers
- laser therapy with Er:YAG lasers
- laser therapy with surgical diode lasers
- risks and side effects
- the importance of correct diagnose
- cancer
- cytogentic effects?
- a false picture of health
- tiredness
- pain reaction
- do high doses of laser therapy damage tissue?
- is it only an effect of temperature?
- protection against radiation injury
- how to measure effects of laser therapy
- thermography
- magnetic resonance imaging
- high resolution digitized ultrasound B-scan
- tensile strength
- other objective methods
- does it have to be a laser?
- FDA (Food and Drug Administration)
- how well documented?
- confused?
- the funding research
- as time goes by
- Medical indications
- who and what can be treated?
- acne
- allergy
- antibiotic resistance
- arteriosclerosis
- arthritis
- asthma
- blood preservation
- blood pressure
- bone regeneration
- burning mouth syndrome
- cancer
- cardiac conditions
- carpal tunnel syndrome
- cerebral palsy
- crural and venous ulcers
- delayed onset muscular soreness (DOMS)
- depression, psychosomatic problems
- diabetes
- duodenal/gastric ulcer
- epicondylitis
- erythema multiform major
- fibrositis/fribomyalgia
- headache/migraine
- heamorrhoids
- herpes simplex
- immune system modulation
- inflammation
- inner ear conditions
- laryngitis
- lichen
- low back pain
- mastitis
- microcirculation
- morbus sluder
- mucositis
- muscle regeneration
- mycosis
- nerve conduction
- nerve regeneration and function
- oedema
- ophthalmic problems
- pain
- periostitis
- plantar fasciitis
- salivary glands
- sinuitis
- spinal cord injuries
- snake bites
- sports injuries
- stem cells
- stroke, irradiation of the brain
- tendinopathies
- tinnitus, vertigo, meniere's disease
- tonsillitis
- trigeminal neuralgia
- thrombophlebitis
- tuberculosis
- urology
- warts
- wiplash-assosiated dissorders
- vitiligo
- womens' health
- wound healing
- zoster
- idications in the pipeline
- alzheimer's disease
- botox failures
- cellulites
- cholesterol reduction
- complex reigonal pain syndrom (CRPS)
- eczema
- erectile dysfunction
- familiar amyotrophic lateral sclerosis (FALS)
- glomerulonephritis
- obesity
- orofacial granulomatosis
- Parkinson's disease
- post-mestrual stress
- pemphigus vulgaris
- sleeping disorders
- withdrawal periods
- wrinkles
- consumer lasers
- Dental LPT
- the dental laser literature
- on which patients can LPT be used?
- dental indications
- alveolitis
- anaesthetics
- aphthae
- bleeding
- bisphosphonate related osteonecrosis of the jaw
- caries
- dentitio dificilis (pericoronitis)
- endodontics
- extraction
- gingivitus
- herpes zoster
- hypersensitive dentine
- implantology
- leukoplakia
- lingua geographica (glossitis)
- lip wounds
- nausea
- nerve injury
- orthodontics
- mild dental pain
- paediatric dental treatment
- periodontics
- prosthetics
- root fractures
- secondary dentine formations
- temperature caveats
- toemporo-mandibular disorders (TMD)
- TMD and endodontics
- other dental laser applications
- dental pohoto dynamic therapy
- composite curing
- deminerallisation
- tooth bleaching
- caries detection
- lasers as a diagnostic tool
- case reports
- Non Coherent Light Sources
- Veterinary Use
- case reports
- Contra Idications
- pacemakers
- pregnancy
- epilepsy
- thyroid gland
- children
- cancer
- haemophilia
- irradiation of the brain
- radiation therapy patients
- diabetes
- tatoos
- light sensitivity
- Coherence
- the role of coherence in laser phototherapy
- itroduction
- summary
- Dose and Intensity
- basics about energy
- output power
- power density
- the laser beam
- the laser probe
- pulsed lasers
- energy density
- treatment dose
- the dose does not demend on the intensity
- dose per point
- more about treatment technique
- The Mechanisms
- are biostimulative effects laser specific?
- is it possible to prove that laser therapy doesn't work?
- comparisons between coherent and non-coherent light
- what is the importance of the length of coherence
- hode's hamburger
- hode's big burger
- abrahamson's apple
- moonlight
- how deep does light penetrate tissue?
- bright light phototherapy
- similarities and differences
- possible primary mechanisms
- polarisation effects
- what characterises the light in a laser speckle
- porphyrins and polarised light
- cell cultures and tissue have different optical properties
- tthe effect of heat development in the tissue
- macroscopic heating
- the microscopic heat effect
- mechanical forces
- excitation effects
- primary reactions due to excitation
- secondary reactions due to cell signaling
- flourescence-luminescence
- multi-photon effects
- llasting effects in tissue
- non-linear optical effects
- opto-acoustic waves
- secondary mechanisms
- effects on pain
- effects on blood circulation
- stimulatory and regulatory mechanisms
- effects on the immune system
- other interesting possibilities
- summary of mechanisms
- diagnostics with therapeutic lasers
- photodynamic therapy - PDT
- other medical uses of lasers
- A Guide for Scientific Work
- methodology of a trial
- parameters
- technical parameters
- treatment parameters
- medical parameters
- closer description of the technical parameters
- name of instrument (producer)
- laser type and wavelength
- laser beam characteristics
- number of sources
- beam delivery system
- output power
- power density at probe aperture
- calibration of the instrument
- closer description of the treatment parameters
- treatment area
- dose: energy density
- dose per treatment and total dose
- intensity: power density
- treatment method
- treatment distance (spot size), type of movement, scanning
- sites of treatment
- number of treatment sessions
- frequency of treatment sessions
- closer description of the medical parameters
- description of the problem to be treated
- patients (number, age, sex)
- exclusion criteria
- inclusion criteria
- condition of patient
- pre-, parallel-, or post-medication
- treated with other methods before
- drop-out rates
- follow up
- outcome measures
- statistical analysis
- economy
- gallium-alluminium and all that
- recommendations of WALT - the world assosiation for laser therapy
- The Laser Phototherapy Literature
- the importance of reporting all laser parameters - even in the abstract
- diclofenac, dexamethasone or laser phototherapy?
- another pithole in LPT research
- database of abstracts of reviews of effects (DARE)
- the wound healing contradiction
- wikipedia
- poor documentation - compared to what?
- LPT equipment and the future
- english language books od LPT:
- books in other languages, with ISBN
- laser phototherapy journals
- information for your patient
Original Source: http://www.coldlasers.org/lllt-books/
A Practical Handbook Laser Acupuncture Successful Treatment Concepts
Volkmar Kreisel and Michael Weber - 2012 (Book) 4330
This book is our top recommendation for any acupuncture style cold laser treatment protocols. It is beautifully written and illustrated.
View Resource

High-Tech Acupuncture with Laser Light
- an equisite light therapy
- biostimulation
- light can heal
- primary stimulation effects
- secondary simulation effects
- is ther optimum stimulation
- laser acupuncture
- high-tech and tradition
- laser ear acupuncture
- is there an optimum dose
- resonance therapy
- what is resonance therapy
- explanatory models
- laser frequencies and those who discovered them
- resonance theapy on the ear
- additional methods and synergisms
- suplementary acupuncture methods
- special applications
- synergisms
- laser types
- laser types by wavelength
- laser types by type of signal
- laser types by form of application
- laser classes
Practical Guidelines
- point localization
- selecting the frequency
- recommended doses
- laser acupuncture: doses and treatment time with laser pen and laser needle
- area therapy: dose and treatment time with laser shower and dermaspot
- important information regarding therapy plans
- containdications and side effects
Treatment Concepts
- orthopedics
- achillodynia
- arthitis, idiopathic juvenile (pediatric rheumatism)
- arthritis, rheumatoid (chronic polyarthritis)
- arthritis urica (gouty arthritis)
- aseptic osteonecrosis
- bakers cyst (popliteal cyst)
- slipped disk (spinal disk herniation)
- bursitis
- chrondophathia patellae
- coxarthosis (arthosis of th hip)
- CRPS (complex regional pain syndrome, Sudeck's disease, reflex dystrophy)
- epicondylitis humeri
- exostosis (bony outgrowth)
- heel spur (calcaneal spur)
- fibromyalgia
- gonarthosis (arthosis of the knee joint)
- hallux valgus (hallux rigidus, bunion)
- cervical spine syndrome
- sacroiliac joint blockage (SIJ blockage)
- capsular ligament injury
- lumbosciatica (sciatica syndrome, irritation of the nerve root)
- lymphatic edema, postoperative
- metataralgia
- muscle fiber rupture (traumatic myopathy)
- Myofascial pain syndrome
- shoulder-arm syndrome
- spinal canal stenosis
- wound healing disorder
- neurology
- carple tunnel syndrome CTS (median nerve compression syndrome)
- cephalgia
- facial paresis
- migraine
- multiple sclerosis MS (encephalomyelitis disseminata)
- paresis (incomplete paralysis)
- Parkinson's syndrome (Parkinson's disease)
- phantom pain
- polyneuropathy
- restless leg syndrome RLS
- transient ischemic attack TIA (stroke)
- psychosomatic disorders
- anorexia nervosa
- burnout syndrome
- depression
- jet lag (dysrhythmia)
- concentration disorders
- addictions - alcohol abuse
- addictions - nicotine abuse
- throat, nose and ear
- otitis media (inflammation of the middle ear)
- parotitis
- acute sinusitis
- chronic sinusitis
- tinnitus
- tonsillitus (angina tonsillaris)
- internal medicine
- allergic disorders - basic laser desensitization
- allergic disorders - allergic exanthema
- allergic disorders - hay fever
- allergic disorders - food allergies
- angiopathies - chronic venous insufficiency CVI
- angiopathies - hemorrhoids
- angiopathies - raynaud's disease
- angiopathies - thrombophlebitis
- gastrointestinal disorders - ulcerative colitis
- gastrointestinal disorders - gastritis
- gastrointestinal disorders - hepatitis
- gastrointestinal disorders - crohn's disease
- lung disorders - bronchial asthma
- lung disorders - acute bronchitis
- lung disorders - chronic bronchitis
- lung disorders - COPD (chronic obstructive pulmonary disease)
- metabolic disorders - diabetes mellitus
- dematology
- acne (acne simplex)
- atopic eczema / neurodermatitis
- hyperhidrosis
- psoriasis
- seborrjeic eczema
- pediatrics
- adenoids (adenoid vegetations, polps, palatine tonsil)
- attention deficit hyperactivity syndrome ADHS
- attention deficit syndrome (concentration disorder)
- abdominal pain, functional
- chronic bronchitits
- three months' colic (regulation disorder / infant crying)
- enuresis nocturna (bedwetting)
- whooping cough (petussis)
- tympanic effusion (tubal catarrh)
- obesity (adipositas)
- underweight (growth disorder)
- cerebral paresis (cerbral palsy)
- gynaecology
- mastitis (inflammation of the mammary glands)
- PMS (postmenstral syndrome)
- morning sickness (hyperemesis gravidarium)
- dental medicine
- stomatitis/gingivitis/aphtea
- tooth extractions
- bleeding gums
- toothache
- ophthalmology
- age-related macular degeneration AMD
- central serous chorioretinopathy (central serous retinitis)
- glaucoma
- conjuctivitis
- retinitis pigmentosa
- dry eyes (sicca syndrome)
Original Source: http://www.coldlasers.org/lllt-books/
Handbook of Photomedicine
Michael R. Hamblin, PhD and Ying-Ying Huang, MD - 2014 (Book) 4333
The Handbook of Photomedicine includes info on all types of medical lasers used in medicine including LLLT, surgical usage and photodynamic therapy (PDT).
View Resource

History and Fundamentals
- introduction: historical vignettes from the feild of photomedicine
- history and fundamentals of lasers and light sources in photomedicine
- light-tissue interactions
- history and fundamentals of photodynamic therapy
- history and fundamentals of low-level laser therapy
Diseases Caused by Light
- uv effects on the skin
- photocarcinogenesis nonmelenoma skin cancer
- autoimmune photodermatoses
- photoaging
- uvr-induced immunosurpression
- the porphyrias
- photoprotection
- botanical antioxidants for photochemoprevention
- reversal of DNA damage to the skin with DNA repair liposomes
- climate change and ultraviolet radiation exposure
- photochemistry and photobiology of vitamin D
Ultraviolet Phototherapy
- phototherapy for psoriasis
- PUVA therapy
- extracorporeal photopheresis
- ultraviolet C therapy for infections
Photodynamic Therapy (PDT)
- recent advances in developing improved agents for photodynamic therapy
- 5-aminolevulinic acid and its derivatives
- genetically encoded photosensitizers: structure, photosensitization mechanisms, and potential application to photodynamic therapy
- light dosimetry for photodynamic therapy: basic concepts
- multimodality dosimetry
- cell death and PDT-based photooxidative (phox) stress
- vascular and cellular targeted PDT
- photodynamic therapy for increased delivery of anticancer drugs
- targeting strategies in photodynamic therapy for cancer treatment
- enhancing photodynamic treatment of cancer with mechanism-based combination stratagies
- nanoparticles for photodynamic cancer therapy
- drug delivery stratagies for photodynamic therapy
- antimicrobial PDT fo clinical infectious diseases
- PDT and the immune system
- detection of bladder cancer by fluorescence cystocopy: from bench to bedside the hexvix story
- photochemical internalization: from bench to bedside with a novel technology for targeted macromolecule therapy
- the story of tookad: from bench to bedside
- photodynamic therapy in ophthalmology
- photodynamic therapy in dermatology
- photodynamic therapy in the gastrointestinal tract
- photodynamic application in brain tumors
- photodynamic therapy for malignant pleural disease
- clinical photodynamic therapy in the Chinese region
- photodynamic therapy and fluorescent diagnostics in the Russian federation
Low-Level Laser (Light) Therapy (LLLT)
- chromophores (photoacceptors) for low-level laser therapy
- low-level laser therapy signaling pathways
- irradiation parameters, dose response, and devices
- low-level laser therapy: clearly a new paradigm in the management of cancer therapy- induced mucositis
- low-level laser therapy for wound healing
- low-level laser therapy in the treatment of pain
- low-level laser therapy in arthritis and tendinopathies
- low-level laser therapy and LED therapy on muscle tissue: preformance, fatigue, and repair
- low-level laser therapy for stroke and brain disease
- low-level light therapy for nerve and spinal cord regeneration
- low-level laser therapy in dentistry
- low-level laser therapy and stem cells
- low-level light therapy for cosmetics and dermatology
Surgical Laser Therapy
- laser and intense pulsed light treatment of skin
- therapeutic uses of lasers in eye care
- lasers used in dentistry
- lasers used in urology
- lasers used in otolaryngology
- laser treatment to nanoparticles for theranostic applications
- laser imminutherapy
- tissue repair by photochemical cross-linking
Other Phototherapies an Future Outlook
- optical guidance for cance interventions
- phototherapy for newborn jaundice
- biological evidence of the efficacy of light therapy in psychiatric disorders
- future developments in photomedicine and photodynamic therapy
Original Source: http://www.coldlasers.org/lllt-books/
Performance Chiropractic and Wellness: The Complete A-Z Manual for Low Level Laser Therapy 5th edition
Jerome Rerucha B.S., C.S.C.S., D.C. - 2015 (Book) 4332
Dr Rerucha is on the cutting edge at documenting how different pulsing frequencies can be used for different stilulatory effects. He works mainly with Erchonia.
View Resource

The Biological Basics of Low Level Laser Light Therapy
- summary
- introduction
- Alexander Gurwitsch: cells emit light
- non-linear dynamics
- introducing quantum physics
- itroduction to quantum biology
- quantum coherence in biology
- biological coherence and the sensitivity of living systems
- Fritz Albert Popp: biophotons
- Guenther Albreecht-Buehler: cells respont to light
- Mae-Wan Ho: visualizing coherence
- conclusions
Therapeutic Laser Applications
- how does low level laser therapy work?
- what are the advantages over other modes of therapy?
- cliniclal use of low level laser therapy
- abstract submitted to laser and surgury medicine
- background and objective
- methods
- results
- conclusion
- safety considerations
- eye considerations
- pace makers and other implanted devices
- pregnancy
- excessive toxicity
- preface to treatment section
Nerver Roots
- flexion and extension
- lateral flexion
- rotation
- MRT (muscle response testing) through ROM of cervical spine
- shoulder
- neurological level
- C5
- C6
- C7
- C8
- T1
- S1
- L5
- L4
- L3
- L3-L5
- L2-L4
- L1-L3
- low back
Top Ten Laser Protocols
- organ / glands / tissue
- acute injury (shock)
- pain
- lymphatic protocol
- detox protocol
- immune protocol
- hormone protocol
- basic cranial nerve
- tissue memory
- trauma preparation protocol
A-Z Laser Protocols
- abdominal cramping
- abdominal inflammation/pain
- abrasions
- abscess
- achilles tear / strain (partial only; not rupture)
- acidosis (hyperacidity
- acid reflux
- acne
- acute injury
- adenoids
- (ADD) atention deficit disorder and hyperactivity disorder (ADHD)
- Addiction
- addison's disease
- adhesions
- adhesive capsulitis
- adrenal
- aids
- allergies
- alopecia
- alpha waves
- alzheimer's
- amenorrhea
- amoebas
- amyotrophic lateral sclerosis / lou gehrig's disease / motor neuron
- amnesia
- anemia
- anger
- angina
- anosmia (loss of smell)
- anxiety appendicitis
- arrhythmias
- arteries / arteriosclerosis
- arthritis
- asthma
- ataxia
- athlete's foot
- atrophy
- backache / back pain
- bacteria
- bed sores
- bedwetting
- bell's palsy
- beta waves
- bites
- bladder
- bleeding gums
- bloating
- blood pressure (high)
- blood pressure (low)
- blood sugar balance
- boils
- bone
- bowel
- bradycardia
- brain
- breast augmentation
- bronchitis
- bruises
- buerger's disease
- bunions
- burns
- burns (second degree)
- bursitis
- calcium deposits or formations
- candida
- canker sores
- capsulitis
- carpal tunnel syndrome
- cartilage
- cataracts
- chemical peels / resurfacing
- chest pain
- chicken pox (herpes zoster / varicella)
- cholecystitis
- cholelithiasis
- chronic fatigue
- chronic pain
- circulation
- cirrhosis
- cold sores (herpes simplex 1)
- colds and flu
- colitis
- concussion
- confusion
- congestion
- congestive heart falure (CHF)
- conjunctivitis (pink eye)
- costipation
- cramps (muscle)
- cranial nerves (general)
- cranial nerves VIII
- crepitus
- crohn's disease
- cuts
- cushing's syndrome
- cytomegalovirus (herpes syndrome V)
- deer tick
- delta waves
- depression
- dermatitis
- detoxification
- diabetes
- diabetic neuropathy
- diabetic ulcers
- digestion
- dim vision
- disc herniation
- dizziness
- dupuytren's contracture
- dyslexia
- ear ache
- ear infection
- eczema
- edema
- emotional stress
- emphysema
- emulsification of fat
- endometriosis
- epistaxis
- epstein - barr virus
- esophagitis
- exercise recovery
- eye conditions
- facet syndrome
- facial paralysis
- fever
- fever blisters
- fibromyalgia
- flu
- food intolerance
- food poisoning
- foot fungus
- fracture
- fungus
- gait
- gallbladder (general)
- gallbladder (stones)
- ganglion cyst
- general musculoskeletal
- gerd
- gingivitis
- glaucoma
- goiter
- gout
- gums
- headache
- heart
- heartburn
- hearing difficulty
- hemorrhoids
- hepatitis A
- hepatitis B
- hepatitis C
- hernia
- herpes simplex
- herpes zoster (chickenpox / varicella)
- HIV
- hives
- hoarseness
- hormone balance
- hot flashes
- human papilloma virus (HPV)
- hyperactivity
- hyper/hypo-tension
- hyper/hypo-thyroid
- hyper/hypo-gycemia
- impotence
- immune enhancement
- incontinence
- indigestion
- infection
- inflammatory bowel disease
- inflammation
- influenza
- injuries
- insect bites
- irritable bowel syndrome
- ischemia
- jaundice
- joints
- keloid
- kidney
- kidey stones
- large intestine
- laryngitis
- ligament
- liposuction
- liver (balace and support)
- loss of smell (anosmia)
- loss of taste
- low back pain
- lungs
- lyme disease
- lymphadentis
- lymphatic
- macular degeneration
- memory problems
- meniere's disease
- meniscus sprain (grade 1)
- menopause
- mensturation
- mental fatigue
- meridian balance 15
- migraine
- motion sickness
- multiple sclerosis
- muscle
- muscle spasm
- myocardial inrarction
- nerve root
- neurogenic inflammation
- neuropathy
- nervousness
- nose bleed
- numbness
- nystagmus
- ocular motility disorders
- ocular nerve
- olfactory nerve
- osgood-schlatter disease
- otitis
- pain
- pain (chronic)
- pain (general)
- injury related pain (localized)
- pain (acute injury)
- pancreas
- parasite
- parasympathetic facilitazation
- paresthesia (numbness)
- periodontal disease
- pink eye (conjunctivitis)
- plantar fasciitis
- pneumonia
- polycystic kidney diseases
- polycystic ovary
- post operative scar revision
- post operative wound healing / pain
- post traumatic stress disorder (PTSD)
- postnasal drip
- premenstral syndrome (PMS)
- pre set head PL-touch
- pre-op
- prostate
- psoriasis
- punctures
- rash
- reflex sympathetic dystrophy (RSD)
- renal problems
- respiratory problems
- restless leg syndrome
- retinitis pigmentosa
- rheumatism
- ringworm
- road rash
- scar tissue
- sciatica
- sedation
- seizures
- shingles
- sinusitis
- skin
- sleep apnea
- small intesine
- smell - lack of
- sore throat
- soreness
- spasm
- spider veins
- spleen
- sprains
- spurs
- standars (neurological) setting
- stanard (up-regulation) setting
- staph infection
- stings
- stomach ulcer
- strep infections
- stress
- stroke
- sty
- subluxation
- sunburns
- swimmer's ear
- swollen ankles
- sympathetic calming
- tachycardia
- taste - lack of
- teeth
- tendonmyopathy (tendonitis)
- tension headaches
- theta waves
- thoratic outlet syndrome
- throat
- thrush
- thyroid (hyper)
- thyroid (hypo)
- tinnitus
- TMJ
- toenail fungus
- tonsilitis
- toothache
- ulcer
- ulcerative colotis
- up-regulation
- urinary tract infection
- varicose veins
- veins
- venereal warts
- viral infections
- voice
- vomiting
- water retention
- watery discharge from eye
- warts
- wounds
- yeast
Original Source: http://www.coldlasers.org/lllt-books/
No Cure from LiteCure
Jan Tunér, Mar 22, 2014 - Annals of Laser Therapy Research (Publication) 4402
This article from Jan Tuner talks about LiteCure's horrible marketing, how 980nm is really bad and how Class 4 lasers are too powerful and often misused. They recommend 905nm for deep penetration.
View Resource
More Lies and Subterfuge from the World of Class IV Laser Therapy
By Jan Tunér
The US laser manufacturer LiteCure (a.k.a. Companion/Pegasus for veterinary version) belongs to a group of laser manufacturers that confuse customers and let consumers pay a high price for something that they do not need. LaserAnnals has previously addressed the so-called Class IV lasers for LPT in general and in a few cases mentioned this particular culprit LiteCure. In this article, we will make a closer check on the credibility and ethics of this company.
Marketing is generally a way of stretching the truth or at least highlighting potential benefits of a product without mentioning the drawbacks. Not very ethical but more or less what consumers expect. Sheer lying is a bit different, and LiteCure uses blatant lies in its marketing. Let us see the first lie:
Lie #1. LiteCure originally claimed that 980 nm has a much better penetration than 808 nm, and that the very high output of their lasers improves the penetration. The illustration below is from their early attempts at marketing the supposed benefits of their device:
Anyone with some basic knowledge about tissue optics knows that 980 nm has a poor penetration due to absorption by water and lipids, and that 808 nm (the illustration actually states 880 nm, but this is not a commonly-used laser wavelength so we assume this was another error…) actually is in an optical window where penetration through skin is optimal. Using very high power with 980 nm doesn’t increase penetration considerably, but instead causes more light to be absorbed superficially more quickly, leading to heat generation. And LPT is not based upon heat but upon stimulation!
Knowledgeable scientists, experienced clinicians and other manufacturers were quick to criticise, however, and to call LiteCure out on this lie, and over time LiteCure has responded by adding the deeper-penetrating 810 nm wavelength to their products, and by modifying the image, as follows:
Although a step in the right direction, even this illustration is still misleading and, basically, incorrect: The effective depth of laser irradiation does not increase over time.
Further to that, the “effortless” non-contact technique causes considerable energy loss by reflection and backscatter – together, remittance, which has been measured at upwards of 80% from bare skin (Al Watban, 1996) – and up to 100% energy loss due to absorption within animal hair/fur. This is hardly “efficient”!
The truth is the opposite to what their sales claims try to tell: A 0.5 W 808-810 nm Class 3B laser actually has a superior ability to penetrate into the body, whereas a 10.0 W 980 nm Class 4 has limited ability and also causes more problems with regards to heat generation. And, as the lower-powered Class 3B device may be applied in contact with the skin directly over the pathological tissue, and held steady for the necessary time to deliver the appropriate amount of energy, it is also significantly more efficient, accurate and safe.
The problem is that their consumer group is rather ignorant about LPT basics and swallow the bait. Fortunately for LiteCure, very high energies are bio-inhibitory and have a temporary pain relieving effect. This is an impressing effect when demonstrated. The downside of the procedure is that the needed reduction of an inflammatory process in inhibited and so is the body’s ability to regenerate itself. This is what is called “a sales trick”.
Lie #2. In its advertising material the LiteCure company writes: “World renowned Laser Therapy Experts, Jan Tunér and Lars Hode have indicated the advantages of high power laser therapy. The (research) literature supports the hypothesis that higher power density yields better clinical results.”
This is similar to the way the devil reads the bible. The above conclusion follows a part of our book where the remarkably low powered lasers on the Canadian market in the ‘90s is discussed. The vast majority of the lasers used were HeNe 1-2 mW and GaAlAs 5-30 mW. So the 400 mW lasers that had just arrived on the market at that time seemed to have a new potential – and they had.
Continued reading of our book reveals that high energies probably will have a better effect on pain conditions but probably not on superficial conditions such as wound healing. In fact, the discussion following the text about “high power” strongly modulates their usefulness.
This text appeared initially in the 2002 book “Low level laser therapy – clinical practice and scientific background”. In following versions of this book, the text has been modified and becomes more critical of extreme energies. And believe me, the next one will be even more critical, to avoid any misunderstandings.
Read my lips: “Tunér and Hode do not recommend 15 W Class IV lasers, not even 5 W!”
An appropriately configured and applied Class 3B device can do all that we need, and if you want to reach deep targets the 904 nm superpulsed GaAs is the best tool!
LiteCure type of science
Recently a LiteCure research paper on fibromylaglia (FM) was published:
Panton L, Simonavice E, Williams K, Mojock C, Kim JS, Kingsley JD, McMillan V, Mathis R. Effects of Class IV laser therapy on fibromyalgia impact and function in women with fibromyalgia. J Altern Complement Med. 2013 May;19(5):445-52.
FM is a devastating condition and LPT is probably a viable option to use, especially since other therapies are rather ineffective and life-long intake of painkillers not a viable option, with the side effects in mind. The study by Panton is obviously performed by a competent team of medical experts, but it seems they have “been taken for a ride” by the LiteCure company. The overall effect of the laser treatment was modest, but had some effects.
So let us have a look on this paper…
For the laser group, treatment was rendered utilizing a LCT-1000 (LiteCure LLC, Newark, DE) solid-state GaAlAs laser delivering a continuous-wave, dual-wavelength laser with 20% 810 nm, and 80% 980nm at 10 W. Each 56.45 cm2 treatment point was treated with laser at 10.63 J/cm2 and warm air utilizing a grid scanning technique to avoid overheating tissue. Participants were instructed to expect some warmth but that the treatment should not burn and to provide verbal cues if the treatment spots became excessively warm. Each treatment point was treated for exactly 60 seconds for a total of 600 J per point, for a total daily treatment dose of 4200 J. The dual wavelength was used for two reasons: (1) this is what is commercially available and (2) two wavelengths allow for treatment in patients with different skin colours since different melanin concentrations will absorb light differently. Both wavelengths are in the accepted therapeutic window. The sham treatment consisted of 60 seconds of warm air alone over the seven tender points.
Now, let us try to make some sense about this study:
a. The cause of FM is not known, but it is manifested by painful bodily points. If pain were a separate biological unit, smashing it with a sledge hammer might be useful. But there is probably more to it, like peripheral neural sensitisation and inflammation. 600 J (!) is given to each point and this is a very high and quite inhibitive energy. And a “point” is declared to be 56.45 cm2. This is rather an area. But by spreading out the light over a large area, the dose becomes 10.63 J/cm2. Such a dose appears to be reasonable, but the energy is not.
b. The paper says: Like the IIIB lasers, recently developed Class IV therapeutic lasers use diffuse light at wavelengths in a therapeutic window that allow penetration of the light deep into the tissue. True, but these lasers do not penetrate deeper than the Class IIIB/3B lasers, so this is a deliberately misleading statement. Further, Class IV/4 therapeutic lasers are not exactly “recently developed”: The defocused beams of Class IV/4 surgical lasers have been used for therapy for equally as long as Class IIIB/3B devices. And the first commercially-available dedicated Class IV/4 therapeutic lasers came on the market in Europe during the ‘90s – which, of course, contradicts the claims by LiteCure and others that Class IV/4 laser therapy is new improvement of Class IIIB/3B. As they are now, these earlier Class IV/4 therapeutic lasers were very expensive and inefficient, and proved no more effective than the already-available lower-powered lasers, so their use did not flourish until the marketing machine took hold in the USA.
c. The paper says: This development has led to the use of Class IV lasers to treat a variety of conditions including skin lesions(24,25), acute soft-tissue injuries (26), and chronic pain syndromes (27) such as FM. In fact, the references 24-27 are not related to the use of “Class IV” LPT lasers at all! This is a technique used often by LiteCure and other marketers of high-powered Class IV therapeutic lasers, banking on the fact that the casual reader will not follow through and actually read the referenced studies.
d. The paper says: There are only a few studies that have used laser therapy to treat pain (16,17,27,37,38). What about 125 published RCTs? If changed to “FM pain”, this is a more valid statement. And one of the most frequently quoted papers on FM and LPT (Gür et al.) used 2 J per point and with better results.
e. The paper says: Studies suggest that Class IV lasers have a beneficial analgesic and anti-inflammatory effect in humans (47-50). No, they don’t! All four papers to which they’ve referred are on Class 3B!
f. Previous studies on FM and LPT have been using considerably lower energies, so the reason for increasing these by a factor 100 seems to have but one background: To prove the superiority of the manufacturer’s product. However, the clinical outcome of this paper was not better than those where is Class 3B lasers have been used.
And let’s address another niggling falsehood: There is no such thing as “Class IV technology”!! 499 mW is Class 3B, 501 mW is Class IV. This is no “technology”. Laser classification is simply related to the relative risk posed by the power, wavelength and distribution of the laser emission!
The manufacturers of the Class IV lasers used in LPT have sponsored a small number of clinical studies. They all contain considerable flaws and even lies and are far from convincing. But they do contribute to the general confusion and are an obstacle in the general acceptance of laser phototherapy.
As mentioned previously, a typical trick of the Class IV vendor is to make reference to Class 3B papers, with proper documentation of their own products lacking. This was the old trick of LED vendors in the ’90s. The LEDs have, in the meantime, created their own scientific groundwork and do not have to use sales tricks any longer.
You can stop reading here, but if you like, here is the actual text from the book that is supposed to recommend Class IV lasers:
Stronger = better?
The power output of therapeutic lasers has increased radically during the nineties. McKibbin reports that there were about 1800 therapeutic laser units in Canada in 1990. 22% of them were HeNe lasers with an output of 1 mW or less, 35% HeNe lasers with 1-2 mW, 13% 830 nm units with an output up to 5 mW, 3% 830 nm units with an output up to 30 mW, 26% GaAs units with an output of 5 mW or less, and 1% units in the 760-780 range nm with an output up to 30 mW.
Now in 2009, the situation is quite different. HeNe units are being replaced by stronger InGaAlP lasers up to 500 mW, GaAlAs units of 7 000 mW are on the market, and GaAs units of 100 mW and more are available.
Even though it is possible to attain some effects with a 1-2 mW laser, there is no doubt that with a laser 100 times stronger, it is much easier to achieve biostimulating effects, at least if one intends to use treatment periods of the same length. Power density is also very important!
The authors used to have certain misgivings about an “inflation” with respect to the output power of therapeutic lasers. One misgiving was, and still is, the obvious risk of eye damage. The need for protective glasses has previously been exaggerated, but is now becoming more important. Another misgiving is the lack of research in the field of “high-power” therapeutic lasers. So far, insufficient data have been published on these powerful lasers. For the moment, we must rely primarily on our own clinical experience. That experience, however, is so encouraging that it cannot be ignored, even with the lack of scientific support. It would appear that “high-powered” therapeutic lasers will be able to further expand the scope of laser therapy, especially in pain therapy.
The doses previously recommended for laser therapy still hold true, in a way. However, much of what we know about dosage is based upon wound healing studies. This is the field in which both stimulating and inhibiting doses have generally been observed. But a wound is superficial, and the superficial tissue will absorb most of the laser energy. So treating a condition in the inner ear through the bone behind the ear is quite a different matter. The dense bone behind the ear absorbs some 90% of the light energy. Skin and blood absorb another 5%. Thus, 100 J in contact mode means only some 5 J or less in the inner ear. For pain and inflammation in large joints, such as the knee, quite a few joules may be required on the surface before the actual target receives the energy needed.
Using the same amount of energy but with different energy densities will not necessarily trigger the same biological response. Kim [545] used 1.2 J in plastic and aesthetic surgery. The energy was delivered either by a 1000 mW or a 60 mW 830 nm laser (1000 mW × 1.2 sec or 60 mW × 200 sec). Both were effective, but the 60 mW laser was more effective in the initial period of wound healing, while the 1000 mW laser was more effective in the late period.
Are strong lasers better than weaker ones?
YES and NO. Output power should not be too low for its purpose. If the power is too low, it causes unnecessarily long treatment time in order to achieve the required total dose (see more about the dose in the next chapter). Also, if output power is too low, it could result in the power density being too low which is an important parameter in treatment. Nor should output power be too high for its purpose. If the power is too high, the light could burn tanned, coloured skin, tattoos or skin with dark hair. Furthermore, in most countries, there is a power limit of 500 mW (= 0.5 watt), above which the laser may be a Class 4 laser. If so, it usually means that it requires oversight by an MD or DDS, more safety measures, and significantly more regulatory control. Also, if the power is too high, it can result in unintentionally high doses which can give less good treatment results than necessary (see the Arndt-Schulz curve in the next chapter). And finally, time is also an important treatment parameter. Administering a certain number of joules over a certain area using a certain laser power during a certain time, may not give the same result as using a ten times stronger laser during one tenth of the time with unchanged optical configuration. Another way to say this is that the rule of reciprocity is not valid. Some laser companies claim that a Class 4 laser ‘by default’ is better than a Class 3B laser (4 is higher than 3, so it has to be better… right?). This is simply not true. The classification of lasers is a measure of eye hazard, nothing else. While defocused Class 4 lasers may well be used successfully in laser therapy, this does not have anything to do with the laser classification.
Original Source: http://www.laserannals.com/2014/03/22/no-cure-from-litecure/
Efficacy of low level laser therapy and intramuscular electrical stimulation on myofascial pain syndrome.
Sumen A, Sarsan A, Alkan H, Yildiz N, Ardic F. - J Back Musculoskelet Rehabil. 2015;28(1):153-8. (Publication) 379
View Resource
Background: Myofascial pain syndrome (MPS) which is an important cause of musculoskeletal pain has shown a dramatic increase in recent years.
Abstract: PMID: 25061034 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Methods: We aimed to evaluate the efficacy of intramuscular electrical stimulation therapy (IMS) and low-level-lasertherapy (LLLT) in patients with MPS.
Results: Patients were randomly divided into three groups. First group were treated with LLLT and stretching exercise. Second group were treated with IMS and stretching exercise. Third group were treated with only stretching exercise. The patients were evaluated through the pain intensity, pain threshold, cervical joint movement range and the neck disability index parameters.
Conclusions: An improvement was found in all parameters for all groups, except for the pain threshold within the control group at the end of the treatment and one month after the treatment. It was found that pain score was significantly lower in Group 1 and 2 at one month after the treatment compared to Group 3. Similarly, it was found that pain threshold score was significantly higher in Group 2 at one month after the treatment compared to Group 3.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25061034
100 positive double blind studies - enough or too little?
Jan Tunér DDS and Lars Hode - (Publication) 4398
This published editorial directs people to their book that details many of the positive double blind studies.
View Resource
Low Level Laser Therapy still has many critics and is not readily accepted as a natural treatment modality in all countries. One main point emphasized by the critics is the lack of scientific documentation. While this was a valid point in the 80s and partly in the beginning of the 90s, is it still a solid argument? There are more than 2000 published studies and the vast majority of these report positive biological effects from Low Level Laser Therapy (LLLT).
The heart of a scientific documentation is the double blind clinical studies. There are some 140 such studies in the field of LLLT and it may come as a suprise to many critics that more than 100 of these are positive. In fact, even most advocaters of LLLT are unaware of this fact. The aim of this Editorial is to disseminate this information to the LLLT community.
Some of the negative double blind studies are well designed and should be taken seriously. Certainly all indications and all parameters cannot work. However, a number of the often quoted negative double blind studies suffer from flaws of several kinds. Some of this is outlined on http://www.laser.nu/lllt/LLLT_critic_on_critics.htm which is a chapter from our recent book "Low Level Laser Therapy - clinical practice and scientific background"
A closer analysis of 100 positive double blind studies will be presented at Laser Florence '99 (October 28-31) and will also appear in the EMLA Millennium laser book.
A weakness in the list is that many double blind studies have only been identified in the abstract form. They may have been published in full at a later stage, but not found by us. 14 studies have only been found as references in reference lists and these have not been found in spite of intensive efforts. For a complete analysis of the 100 positive double blind studies we need the assistance of the visitors of LaserWorld. In the following list abstracts are marked in red and studies not found marked in green. If you have any information about the green studies please contact us. And if you know that an abstract has been published in a journal, please do likewise. The more complete the list is, the better for the LLLT community.
The studies published in journals are listed in full in the book mentioned above.
Atsumi K et al. Biostimulation effect of low-power energy diode laser for pain relief. Lasers Surg Med. 1987; 7: 77.
Barabas K et al. Controlled clinical and experimental examinations on rheumatoid arthritis patients and synovial membranes performed with neodym phosphate glas laser irradiation. Proc. 7th Congr Internat Soc for Laser Surg and Med, Munich June 1987. Abstract no 216a.
Boerner E et al. Double-blind study on the efficacy of the lasertherapy. SPIE Proc. 1996. Vol. 2929: 75-79.
Cheng R. Combined treatments of electrotherapy plus soft laser therapy has synergistic effect in pain relief and disease healing. Surgical and Medical Lasers. 1990; 3 (3): 135
Cieslar G et al. Effect of low-power laser radiation in the treatment of the motional system overloading syndromes. SPIE Proc. Vol 3198. 1997, pp. 76-82.
Emmanoulidis O et al. CW IR low-power laser application significantly accelerates chronic pain relief rehabilitation of professional athletes. A double blind study. Lasers Surg Med. 1986; 6: 173.
Haruki E, Yamaguchi S. Double blind evaluation of low energy laser treatment for painful disease. J Phys Med. 1995; 6: 60-67. (In Japanese with English abstract)
Hopkins G O et al. Double blind cross over study of laser versus placebo in the treatment of tennis elbow. Proc Internat Congr on Lasers, "Laser Bologna". 1985: 210. Monduzzi Editore S.p.A., Bologna. Hoshino H et al. The effect of low reactive level laser therapy in the field of orthopedic surgery. Chronic Pain. 1994; 13: 101-109. (In Japanese with English abstract)
Hoteya K et al. Effects of a 1 W GaAlAs diode laser in the field of orthopedics. In: Meeting Report: The first Congress of the International Association for Laser and Sports Medicine. Tokyo, 1997. Laser Therapy 1997; 9 (4): 185.
Kamikawa K et al. Double blind experiences with mid-Lasers in Japan. 1985. Proc Int Congr on Lasers, "Laser Bologna". 1985: 165-169. Monduzzi Editore S.p.A., Bologna.
Kim J W, Lee J O. Double blind cross-over clinical study of 830 nm diode laser and 5 years clinical experience of biostimulation in plastic & aesthetic surgery in Asians. Lasers Surg Med. 1998; Suppl. 10: 59.
Kinoshita F et al. Clinical evaluation of low-energy, semi-conductor laser therapy in oral surgery - a double blind study. Josai Shika Daigaku Kiyo (Bulletin of Josai Dental University). 1986; 15 (3): 735-742. (in Japanese with English abstract)
Kosaka R et al. Double blind study of low energy diode laser irradiation for chronic pain disorders. J Phys Med. 1993; 4: 156-160.
Kouno A et al. The evaluation of pain therapy with low powerlaser- Comparative study of thermography and double blind test. Biomedical Thermology. 1993; 13: 102-107.
Lonauer G: Controlled double blind study on the efficacy of HeNe-laser beams versus HeNe- plus Infrared-laser beams in the therapy of activated osteoarthritis of finger joints. Clin Experim Rheuma. 1987; 5 (suppl 2) : 39
Lucas C et al. Low level laser therapy bij decubitus statium III. Rapport Hoegschool van Amsterdam. 1994.
Mach E S et al. Helium-Neon (Red Light) Therapy of Arthritis. Rhevmatologia, 1983; 3: 36. (In Russian)
Mester A: Biostimulative effect in wound healing by continous wave 820 nm laser diode. Double-blind randomized cross-over study. Lasers in Med Science, abstract issue July 1988, No 289.
Miyagi K. Double-blind comparative study of the effect of low-energy laser irradiation to rheumatoid arthritis. In: Current awareness of Excerpts Medica. Amsterdam. Elsevier Science Publishers BV. 1989; 25: 315.
Mokhtar B et al. A double blind placebo controlled investigation of the hypoalgesic effects of low intensity laser irradiation of the cervical roots using experimental ischaemic pain. Proc. Second Meeting of the International Laser Therapy Assn., "London Laser", Sept 1992, p 61. Mokhtar B et al. The possible significance of pulse repetition rate in lasermediated analgesia: A double blind placebo controlled investigation using experimental ischaemic pain. Proc. Second Meeting of the International Laser Therapy Assn, "London Laser" Sept 1992. p 62
Neuman I et al. Low energy phototherapy in allergic rhinitis and nasal polyposis. Laser Therapy. 1996. 1: 37.
Palmgren N et al. Low Level Laser Therapy of infected abdominal wounds after surgery. Lasers Surg Med. 1991; Suppl 3:11.
Poliakova A G., Gladkova N D, Triphonova T.D. Laserpuncture in patients with rheumatoids arthritis. Abstracts of ICMART '97 International Medical Acupuncture Symposium, Nicosia, Cyrprus, March 26-29 1997.
Rochkind S et al. Double-blind Randomized Study Using Neurotube and Laser Therapy in the Treatment of Complete Sciatic Nerve Injury of Rats. Proc. 2nd Congr World Assoc. for Laser Therapy, Kansas City, 1998.
Roumeliotis D et al. 820nm 15mW 4J/cm2, laser diode application in sports injuries. A double blind study. Proc. Fifth Annual Congress British Medical Laser Ass. 1987.
Ryo E et al. Double blind test of low energy laser radiation treatment. Evaluation of effectiveness for shoulder stiffness, arthralgia etc. Pain Clinic. 1986; 7: 185-192. (In Japanese with English abstract)
Saeki N et al. Double blind test for biostimulation effects on pain releif by diode laser. 1989. Laser Surgery; 1066: 93-100.
Sasaki K et al. A double-blind controlled study on free amino acid analysis in CO2 laser burn wounds in the mouse model following doses of low incident infrared (830 nm) diode laser energy. Proc. 2nd Meeting if the Internat Laser Therapy Assn., London, 1992, p.4.
Sato K et al. A double blind assessment of low power laser therapy in the treatment of postherpetic neuralgia. Surgical and Medical Lasers. 1990; 3 (3): 134.
Scudds R A et al. A double-blind crossover study of the effects of low-power gallium arsenide laser on the symptoms of fibrositis. Physiotherapy Canada.1989; 41: (suppl 3): 2.
Taghawinejag M et al. Laser-Therapie in der Behandlung kleiner Gelenke bei chronischer Polyarthritis. Z Phys Med Baln Med Klin. 1985; 14.
Tsurko V V et al. Laser therapy of rheumatoid arthritis. A clinical and morphological study. Ter Arkh. 1983; 55 (7) 97-102. (Russian).
Umegaki S et al. Effectiveness of low-power laser therapy on low back pain. Double blind comparative study to evaluate the analgesic effect of low power laser therapy on low-back pain. The Clinical Report. 1989; 23: 2839-2846. (In Japanese with English abstract)
Vélez-Gonzalez M et al. Treatment of relapse in herpes simplex on labial and facial areas and of primary herpes simplex on genital areas and "area pudenda" with low power HeNe-laser or Acyclovir administred orally. SPIE Proc. 1995; Vol. 2630: 43-50
Willner R et al. Low power infrared laser biostimulation of chronic osteoarthritis in hand. Lasers Surg Med. 1985; 5: 149.
Wylie L et al. The hypoalgesic effects of low intensity infrared laser therapy upon mechanical pain threshold. Lasers Surg Med. 1995; Suppl 7: 9.
Yamaguchi M et al. Clinical study on the treatment of hypersensitive dentine by GaAlAs laser diode using the double blind test. Aichi Gakuin Daigaku Shigakkai Shi - Aichi-Gakuin Journal of Dental Science. 1990; 28( 2): 703-707. (in Japanese)
Yoh K et al. A clinical trial for treatment of chronic pain in orthopedic diseases by using 150 mW diode laser system. Result of double blind test. Chronic Pain; 13: 96-100.(In Japanese with English abstract)
Original Source: http://www.laser.nu/lllt/lllt_editorial3.htm
Quantitative In Vivo Imaging of Tissue Absorption, Scattering, and Hemoglobin Concentration in Rat Cortex Using Spatially Modulated Structured Light
David J. Cuccia, David Abookasis, Ron D. Frostig, and Bruce J. Tromberg. - 2009 (Book) 4502
View Resource
12.1. INTRODUCTION
Significant changes in blood flow or in the integrity of cerebral vessels are believed to cause cerebrovascular disease (CVD) and to contribute to dementias including Alzheimer’s disease [1]. Stroke, the most serious form of CVD, is one of the leading causes of death and adult disability worldwide. Acute treatments for stroke, however, are severely limited. Neuroprotective drugs under development show promise at halting the ischemic cascade, but as yet, no such compound has received federal approval in the United States. One of the biggest limitations to this development is the lack of understanding of the mechanisms by which cerebral vessels react to factors such as ischemia, inflammation, blood pressure changes, metabolic demands, and trauma [2]. In order to address these fundamental questions, functional brain imaging techniques such as fMRI and intrinsic signal optical imaging (ISOI) have emerged as tools to visualize and quantify cerebral hemodynamics.
In the neuroscience community, ISOI has long been used to study the organization and functional architecture of different cortical regions in animals and humans [3–5] (see other chapters in this book). Three sources of ISOI signals that affect the intensity of diffusely reflected light derive from characteristic physiologic changes in the cortex. For functional neuronal activation, these have been observed to occur over a range of timescales, including (1) light scattering changes, both fast (over 10 s of milliseconds) and slow (i.e., > ~0.5 s) (2) early (~0.5–2.5 s) absorption changes from alterations in chromophore redox status, i.e., the oxy/deoxy-hemoglobin ratio (known as the “initial dip” period), and (3), slower (~2–10 s) absorption changes due to blood volume increase (correlated with the fMRI BOLD signal). Light scattering changes have been attributed to interstitial volume changes resulting from cellular swelling, organelle swelling due to ion and water movement, capillary expansion, and neurotransmitter release [6,7]. The slower absorption factors have been demonstrated to correlate with the changes in metabolic demand and subsequent hemodynamic cascades following neuronal activation [4,8,9].
Using animal models of acute and chronic brain injury, ISOI has been used to quantify the acute hemodynamic events in response to stroke, including focal ischemia and cortical spreading depression (CSD) [10–21]. Researchers have also used ISOI to locate and quantify the spatial extent of the stroke injury, including ischemic core, penumbra, and healthy tissue zones [18,22]. CSD also plays a key role in migraine headache, and recent laser speckle imaging studies have revealed the neurovascular coupling mechanism to the transmission of headache pain [23,24].
To fully understand the underlying mechanisms in vascular changes associated with cerebrovascular diseases such as stroke, an optical imaging technique that has the capability to rapidly separate absorption from scattering effects can enhance the information content of traditional ISOI, enabling (1) more accurate quantitation of hemodynamic function, (2) isolation of the electro-chemical changes characterized by light scattering, and (3) longitudinal chronic injury studies of function where structural reorganization due to neovascularization can cause significant alterations in scattering [25,26].
Quantitative diffuse optical methods [27] such as spatially-resolved reflectance, diffuse optical spectroscopy (DOS), and tomography (DOT), and diffuse correlation spectroscopy (DCS) possess exquisite sensitivity to these functional and structural alterations associated with brain injury, and have been applied to the study of CSD [11,15,28]. DOS and DOT utilize the near-infrared spectral region (600–1000 nm) to separate and quantify the multispectral absorption (μa) and reduced scattering coefficients (μs′), providing quantitative determination of several important biological chromophores such as deoxy-hemoglobin (HbR), oxy-hemoglobin (HbO2), water (H2O), and lipids. Concentrations of these chromophores represent the direct metrics of tissue function such as blood volume fraction, tissue oxygenation, and edema. Additionally, the scattering coefficient contains important structural information about the size and density of scatterers and can be used to assess tissue composition (exctracellular matrix proteins, cell nuclei, mitochondria) as well as follow the process of tissue remodeling (wound healing, cancer progression). DOS utilizes a limited number of source-detector positions, e.g., 1–2, but often employs broadband content in temporal and spectral domains [29]. In contrast, DOT typically utilizes a limited number of optical wavelengths (e.g., 2–6) and a narrow temporal bandwidth, but forms higher resolution images of subsurface structures by sampling a large number of source-detector “views.” To achieve maximal spatial resolution, the ideal DOT design would employ thousands of source-detector pairs and wavelengths. However, several engineering considerations including measurement time and instrument complexity currently limit the practicality of this approach.
In this chapter we present the basic principles of a new, noncontact quantitative optical imaging technology, modulated imaging (MI) [30–32], and provide examples of MI performance in 2 rat models of brain injury, cortical spreading depression (CSD) and stroke. MI enables both DOS and DOT concepts with high spatial (<1 mm) and temporal resolution (<1 s) in a simple, scan-free platform. MI is capable of both separating and spatially-resolving optical absorption and scattering parameters, allowing wide-field quantitative mapping of tissue optical properties. While compatible with time-modulation methods, MI alternatively uses spatially modulated illumination for imaging of tissue constituents. Periodic illumination patterns of various spatial frequencies are projected over a large area of a sample. The diffusely reflected image is modified from the illumination pattern due to the turbidity of the sample. Typically, sine-wave illumination patterns are used. The demodulation of these spatially modulated waves characterizes the modulation transfer function (MTF) of the material, and embodies the sample optical property information.
12.2. METHODS AND INSTRUMENTATION
12.2.1. Modulated Imaging Spectroscopy
The MI instrument platform was introduced originally by Cuccia et al. [30] Based on this design, we have developed a custom multispectral near-infrared (NIR) MI spectroscopy system capable of imaging between 650 and 1000 nm. A diagram of this system is shown in Figure 12.1.
FIGURE 12.1
Modulated imaging platform. QTH—quartz tungsten halogen; L1—aspheric condenser; H—hybrid hot mirror; DMD—digital micromirror device; L2—projection lens; L3—camera lens; LCTF—liquid crystal tunable (more...)
Broadband NIR illumination is provided by an intensity-stabilized 250 W quartz-tungsten-halogen (QTH) lamp (Oriel QTH Source with Light Intensity Controller, Newport Corporation-Oriel Instruments, Stratford, Connecticut). Light is collimated and refocused with a pair of aspheric F/#0.7 optical lens systems (Oriel Aspherab). A custom-sized 3.5 in square hybrid hot mirror (Reynard Corporation, i.e., R00670-00) was placed between the lenses to limit the illumination to wavelengths below 1000 nm. Light engine optics taken from a digital projector (NEC HT1000) serve to homogenize and direct the light onto a 0.7 in digital micromirror device (DMD Discovery™ 1100 with ALP Accessory Package, ViALUX, Germany). Grayscale spatial sinusoid patterns are projected at 400 Hz using the ViALUX software development toolkit, which generates the necessary pulse-width modulation of binary sub-frames to produce a specified grayscale bit-depth (1–8 bits). Finally, a fixed focal length (f = 100 mm) projection lens illuminates the tissue at a slight angle from normal with a 15 × 25 mm illumination field. Detection was performed at normal incidence using a CRI Nuance™ camera system, which combines a 12-bit CCD camera and a liquid crystal tunable filter (LCTF; λ = 650–1100 nm, Δλ = 10 nm). To avoid specular reflection, crossed linear polarizers are used in the illumination and detection arms. For this system, the former is a 1.5 in diameter NIR linear polarizer (Meadowlark Optics, VLM-200-IR-R) placed immediately after the projection lens, and the first stage of the Nuance LCTF serves as the latter. The DMD, CCD, and LCTF are controlled via USB by a laptop computer, and synchronized using LabVIEW software (LabVIEW 8, National Instruments), enabling fast acquisition of a series of patterns with various spatial frequencies.
12.2.2. SFD Measurement, Calibration, and Modeling
A detailed description of SFD measurement, calibration, and diffusion modeling is provided by Cuccia [32]. In this work, we modeled diffuse reflectance using a transport-based White Monte Carlo (WMC) method [33,34]. Previously, we have found that compared with Monte Carlo, (1) diffusion predictions over- and underestimate low- and high-frequency diffuse reflectance, respectively, and (2) the quantitative accuracy of diffusion degrades with decreasing albedo [32]. Due to the moderate albedo of brain tissue (μs′/μa ~ 10–20), we chose to analyze all brain data with the WMC approach. This homogeneous tissue model is a significant simplification of the multilayered rat brain, and more work is necessary to accurately model this complex system. We discuss further the consequences of our simple model in Section 12.2.5.
12.2.3. Optical Property Inversion Methods
In this chapter, we use two inversion methods to calculate the absorption and reduced scattering from measurements of diffuse reflectance. When high measurement precision is desired, we use a “sweep” in spatial frequency space, producing an overdetermined set of diffuse reflectance measurements, which can be fitted to our WMC forward model predictions using least-squares minimization. This method is performed for all spatially averaged region analysis of optical properties and chromophores. When increased acquisition and/or processing speed is desired, we alternatively use a rapid two-frequency lookup table method based on cubic spline interpolation [32]. This data can be achieved with a minimal 3-phase, single frequency image set (by demodulating and averaging the images to obtain AC and DC amplitude maps, respectively). On typical personal computers this approach is capable of millions of inverse lookup calculations per second, and is therefore used to calculate all high-resolution images including time sequences. The signal-to-noise ratio (and thus the measurement precision) of either approach is limited by the data sampling, with the two-frequency method having a lower precision with the tradeoff of higher acquisition and processing speed.
12.2.4. Spectral Analysis-Chromophore Calculation
The quantitative absorption coefficient is assumed to be a linear (Beer’s law) summation of individual chromophore absorption contributions:
μa(λ)=2.303∑i=13ci?i(λ),
12.1
where ci and ?i(λ) represent chromophore concentrations and molar extinction coefficients, respectively. Using reported extinction coefficients of HbO2/HbR35 and H2O,36 we can invert Equation 12.1 and calculate tissue chromophore concentration separately at each pixel by linear least-squares fitting to the multispectral absorption images. Total hemoglobin (HbT) and oxygen saturation (StO2) can then be calculated as HbT = HbR + HbO2 and StO2= HbO 2/(HbR + HbO2) * 100, respectively.
12.2.5. Optical Property Mapping: Resolution Versus Quantitation
On a pixel-by-pixel basis, diffuse reflectance versus spatial frequency is fitted to the WMC forward model to extract the local absorption and reduced scattering optical property contrast. This process is repeated for each wavelength, resulting in multi-spectral absorption and scattering spectra at each pixel. The measured contrast from discrete absorbers and scatterers on millimeter and submillimeter spatial scales, however, will possess partial volume effects in all three spatial dimensions. This is due to the physical light transport length scales in tissue, limiting the true x-y resolution of optical property contrast to many detector pixels [32]. This phenomenon is not unique to MI, but present in all planar reflectance imaging measurements of turbid media. Absorption and scattering are calculated using a homogeneous reflectance model, extracting a locally averaged sampling of optical property contrast. Based on simulations of the tissue MTF for varying optical properties [32], we expect the resulting image resolution to scale directly with the transport length, l* = (μa + μs′)− 1, and the spatial frequency of illumination. In this chapter, we place quantitative emphasis on average optical properties and chromophores measured over a field of view that is greater than l*. Spatial maps and videos of these parameters are displayed and referred to as “contrast maps,” with the caveat that high resolution features will exhibit degraded quantitative accuracy.
12.2.6. In Vivo Rat CSD Experiments
12.2.6.1. Animal Preparation
MI spectroscopy measurements were performed on an in vivo Wistar rat model with a thinned-skull preparation. All procedures were performed in accordance with approved IACUC protocol guidelines. The animals were anesthetized, placed in a stereotaxic frame, their skulls thinned and glass coverslip applied. This preparation is described in detail by Masino et al. [37] The resulting thinned skulls allowed direct imaging of the cortex over a 5 × 7 mm field-of-view (whisker barrel cortex, centered at the C2 location). In order to investigate the sensitivity of MI toward studying acute cortical injury, we induced cortical spreading depression (CSD) by applying 1 M KCl solution to the surface of the cortex through a perforated section of skull and dura, located approximately 3 mm above the camera’s imaging field.
12.2.6.2. MI Measurement Protocol
For each of three animals, our MI measurement protocol was twofold. Prior to CSD induction, baseline spatial modulation data were acquired at 6 spatial frequencies (3-phase projections each) from 0 to 0.26 mm−1, at 10 nm intervals over the entire range between 650 and 980 nm. Depending on the wavelength, image acquisition times ranged from 200 ms to 4 s, with total spectral imaging time of approximately 30 s per spatial pattern. The entire measurement (34 wavelengths, 3 phases, 6 frequencies) was repeated three times for statistical averaging yielding an entire measurement time of approximately 30 min.
Next, rapid dynamic measurements were performed, beginning 1 min prior to K+Cl− administration. Here, a significantly reduced data set was chosen in order to achieve high temporal resolution. Two spatial frequencies (0 and 0.26 mm−1) were acquired with three phase projection images, as described in Section 12.2.2, at each of four wavelengths (680, 730, 780, and 830 nm). The resulting 12 images took in total 6 s, permitting a repetition rate of 10 measurements per minute. The animals were followed for a period of 10 min for rats 1 and 2, and a period of 30 min for rat 3.
All images in this study were smoothed by 2D convolution with a Gaussian filter function (FWHM = 3 pixels), and baseline repetitions were averaged prior to data processing. Additionally, time-series data were post-processed by smoothing slightly in time (Gaussian FWHM of 2 timepoints = 12 s).
12.2.6.3. Spatial Frequency Sensitivity Analysis
Because of the differential absorption sensitivity at low and high frequencies, optimal optical property separation is achieved when a large range of frequencies is used [31]. In Figure 12.2a, we depict this differential sensitivity using diffuse reflectance (MTF) predictions versus frequency, increasing μa by 100% from 0.02 (black line) to 0.04mm−1 (gray line). This is done for two values of μs′, 0.6 (solid lines) to 1.2mm−1 (dashed lines), simulating a 100% change in scattering. Notice that the low frequencies have a significant reflectance change due to absorption, while high frequency reflectance remains nearly unchanged. Conversely, reflectance changes due to scattering are observed at all spatial frequencies. In Figure 12.2b, we further visualize this by plotting the reflectance sensitivity to 1% changes in absorption and scattering. Whereas DC reflectance is equivalently sensitive to a fractional change in either absorption or scattering, at high spatial frequencies absorption contrast is lost while scattering contrast is retained. For instance, notice that at our maximum measurement frequency of 0.26 mm−1 the reflectance is roughly 24 times more sensitive to scattering compared to absorption (ΔRd = 0.56 μs′ versus 0.024 * 10−3 for μa). This plays an important role in Section 12.3.2 during our discussion of dynamic scattering measurement.
FIGURE 12.2
(a) Reflectance contrast in absorption and scattering covering a typical range of brain optical properties. (b) The frequency-dependent sensitivity to absorption (black line) and scattering (gray line), respectively. Reflectance at fx = 0.26 mm−1 (more...)
In realistic heterogeneous tissues, a tradeoff exists between maximizing the frequency range for optical property accuracy and obtaining similar sampling volumes. As tissue is a low-pass spatial filter, high frequencies are attenuated quickly with depth. Using diffusion-based forward modeling, we have estimated mean sampling depths at 650 nm using measured average background optical properties of brain tissue. This was done by predicting the depth sensitivity to contrast from a planar perturbation in absorption, given a background fluence profile from spatial frequencies 0 and 0.26 mm−1. Based on these results, we observe qualitatively similar depth sampling, with mean depth sampling ranging between 2.5 mm and 1.2 mm (for fx = 0 and 0.26 mm−1, respectively). In all cases maximal sensitivity was found in the first 1–2 mm, where cortical hemodynamic changes occur.
12.3. RESULTS AND DISCUSSION
12.3.1. Baseline MI Spectroscopy
In Figure 12.3a we show a grayscale planar reflectance image of the cortical region of rat 1 at 650 nm. A dotted-line box denotes the region-of-interest (ROI) used for analysis, selected for its uniform illumination and the absence of cerebral bruising. The Monte Carlo-model fitting of spatial frequency data allows calculation of the absorption and reduced scattering coefficients. In Figure 12.3b we show the spatially averaged diffuse reflectance at 650 nm and the corresponding multi-frequency fit. Excellent agreement is observed between measurement data and the model-based fit, with derived μa and μs′ coefficients of 0.033 and 0.70 mm−1, respectively.
FIGURE 12.3
(a) Reflectance map for rat 1, showing the 3.8 × 5.9 mm region chosen for quantitative analysis. (b) Sample MTF reflectance data (squares) and fit (solid line) at 650 nm. (c) Recovered optical property maps (above) and corresponding image histogram (more...)
Analysis of multifrequency reflectance data separately at each pixel results in spatial maps of absorption and reduced scattering contrast. In Figure 12.3c, we plot the μa and μs′ maps recovered at 650 nm for rat 1. Note the strong absorption in the vein region, due to a large absorption by HbR at this wavelength. Below the images, we show histogram distributions of the corresponding quantitative maps above, indicating the degree of spatial variation in recovered optical properties. The mean and standard deviation for the pixel-wise μa and μs ′ were 0.030 ± 0.007 mm−1 and 0.63 ± 0.13 mm−1, respectively. These statistical results are in good agreement with the spatially averaged reflectance fit from Figure 12.3b, suggesting that our simple pixel-wise fitting approach yields optical properties similar to that calculated using a global analysis.
By mapping the absorption coefficient at multiple wavelengths, we can perform quantitative spectral imaging of tissue. In Figure 12.4, we summarize the baseline spectroscopy results for all three animals. In Figure 12.4a we show the μa (left) and μs′ (right) coefficients versus wavelength (circles) recovered from spatially averaged fitting. Data for rat 1 is shown in black (rat 2 in dark gray; rat 3 in light gray). Note the distinct spectral features in absorption, resulting from oxy- and deoxy-hemoglobin (HbO2, HbR), and water (H2O) absorption. The calculated scattering coefficient generally decays with increasing wavelength, and the results from a power law (μs ′ = A·λ(nm) −b, solid lines) fit are shown. A small residual coupling is observed between measured scattering and absorption spectral features. In particular, the scattering at the shortest and longest wavelengths appears to be underestimated by 5–10%, occurring where the corresponding absorption is highest (due to HbR and H2O, absorption features, respectively). Based on our experiments in layered tissue phantoms [38], we believe this effect is primarily due to frequency-dependent probing volumes in the presence of depth-heterogeneous structures.

FIGURE 12.4
(a) Average μa (left) and μs′(right) spectra over entire ROI (circles). HbO 2, HbR, and H2O concentrations are determined by subsequent least-squares fitting (solid lines) of molar extinction coefficients to the absorption. Data (more...)
Simultaneous linear fitting of the absorption to known extinction coefficients yields measures of chromophore concentration. Shown in Figure 12.4a, multispectral fitting (solid line) for rat 1 yields HbO2, HbR, H2O, HbT and StO2 values of 56.3 μM, 33.2 μM, 63.9%, 89.6 μM, and 56.3%, respectively. Tabulated results of chromophore values for all three animals are shown in Figure 12.4b. Lipid absorption near 930 nm was not apparent in the μa spectrum, and when included in the spectral analysis was not found to significantly affect the results. The small absorption “bump” at 900–910 nm is an artifact of imperfect phantom calibration due to the presence of a sharp, strong silicone absorption peak that is present in the phantom.
We note that the solution for chromophore concentration is well-determined when the number of wavelengths is at least equal to the number of chromophores. Therefore, as few as two wavelengths can be used to separate HbO2 and HbR (if a constant value of H2O is assumed). Repeating the above analysis with 780 and 830 nm only (assuming H2O = 65%) yields results for HbO2 and HbR within 10% of those from full spectral fitting. Repeating the above analyses using a simple diffusion-based model provided qualitatively similar results for absorption and scattering spectra, but in general was found to overestimate the absorption coefficient by 10–25%.
Absorption spectra at each pixel can be separately analyzed to yield spatial maps of local HbO2, HbR, and H2O distribution, shown in Figure 12.5. Notice the high concentration of HbR over the large superficial draining vessel (venous) regions, also reflected in the StO2 image, highlighting the effect of tissue oxygen extraction. Conversely, notice that the high albedo regions with less structural detail are highly oxygenated, with StO2 levels between 60 and 70%. Lastly, the H2O map reveals a relatively homogeneous distribution of water.
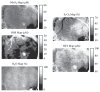
FIGURE 12.5
Chromophore fits to absorption spectra at each pixel yield maps of local HbO2, HbR, and H2O concentration (left). Total hemoglobin (HbT) and oxygen saturation (StO2) maps can then be calculated from HbO2 and HbR.
12.3.2. Dynamic MI Spectroscopy of CSD
We performed measurements of CSD in each of the three rats, as described in Section 12.2.3. The results are presented as follows. We first present data for a single animal, choosing rat 3 for its long observation period of 30 minutes. Three ROIs are selected for analysis, and baseline MI spectroscopy results are reported for each of these regions. Next, the observed dynamic time courses of diffuse reflectance, optical properties, and chromophore concentrations are shown for each ROI. We then present the full spatio-temporal dynamic contrast data for rat 3 (2D + time) in the form of “snapshot” images.
Figure 12.6 summarizes the baseline spectroscopy measurements for rat 3. In Figure 12.6a, we show three regions of interest superimposed on the DC reflectance map, chosen to highlight three different characteristic temporal profiles observed within the field of view. In Figure 12.6b we show the baseline spectral fits for each of these regions, and in Figure 12.6c we tabulate the resulting calculated chromophore concentrations. In general, Region A (black) is a high albedo region lacking any large blood vessels, whereas Regions B (dark gray) and C (light gray) include high-absorption blood vessels and mild cerebral bruising from surgery. These differences are apparent in their recovered absorption spectra and fits, with on average 27% higher HbT, and 32% lower saturation in the vascular regions. Also, 7% higher H2O is found in Regions B and C, which may indicate increased edema due to bruising.
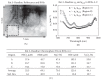
FIGURE 12.6
Regionwise spectral analysis of rat 1 baseline data including the respective (A) ROIs, (B) spectral absorption data (circles) and fit (lines), and (C) tabulated recovered chromophore data for each region
In Figures 12.7–12.9 (for regions A–C, respectively), we present the temporal dynamics of CSD in each ROI of rat 3 as measured by MI. In part (a) of each figure, we plot the multispectral diffuse reflectance changes at fx = 0 mm−1 (DC, top) and fx = 0.26 mm−1 (AC, bottom). In part (b), we plot the recovered Δμa (top) and Δμs′ (bottom) optical properties at each wavelength. While absolute values of diffuse reflectance and optical properties are measured separately at each time point, for visualization purposes all data are displayed as a change from that prior to KCl administration. Absolute optical property values at t = 0 (not shown) demonstrate excellent agreement (~5–10%) with full multifrequency baseline data.
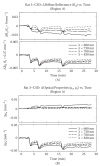
FIGURE 12.7
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx =.26 mm−1, bottom) for Region A of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) coefficients. (more...)
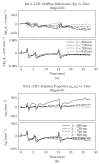
FIGURE 12.8
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx = 0.26 mm−1, bottom) for Region B of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) (more...)
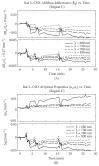
FIGURE 12.9
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx = 0.26 mm−1, bottom) for Region C of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) (more...)
Looking first at the reflectance time courses of Figure 12.7a (Region A), we see in general a series of three CSD events over the 30 minutes, with each transient event occurring for approximately 4.3 minutes. The first event occurs at minute 2.9 after KCl application, indicating an initial latency between the insult and the first resulting spreading depression wave. Reflectance contrast is present in both DC and AC frequency components, but with markedly different signatures. Generally, the DC time course shows a slow, gradual decay, punctuated by sharp, wavelength-dependent spikes/dips (for short/long wavelengths, respectively). Alternatively, the AC signature contains three sets of transient dips consistent across all wavelengths, with final values leveling off progressively lower than baseline. Discussed in detail in the following paragraph, we believe these AC changes are due primarily a result of optical scattering and may be related to neuronal depolarization. The corresponding derived optical properties in Figure 12.7b reflects this, with μs′ trends tracking directly with the measured AC reflectance. As expected, μa trends reveal similar wavelength-dependence of the DC reflectance (with opposite polarity), reflecting changes in HbO2 and HbR.
In Section 12.2.3.3 we noted that the diffuse reflectance at fx = 0.26 mm−1 is 23 times more sensitive to scattering changes compared to absorption. In this context, we propose that the observed magnitude of the CSD-induced AC reflectance changes can only be explained by changes in optical scattering. To concretely illustrate this point, we pick as an example the observed 780 nm AC diffuse reflectance dip in Figure 12.7a at t = 3.7 min of -0.003. Here, the corresponding change in reduced scattering in Figure 12.7b, Δμs′, is calculated to be −0.03 mm−1. In order for this change to instead be due to an absorption-only event, μa would need to increase by 121% from baseline (from 0.038 to 0.084 mm−1). This increase would also need to be accompanied by a drop in Rd (fx = 0 mm−1) of 0.12 (33%), whereas the actual observed DC reflectance only drops by 0.008 (<1%) and thus cannot explain the change. Secondly, we note that the three sets of AC reflectance dips occur consistently across all four wavelengths. While an approximate 120% increase in HbT could induce this decrease at high frequency, it would also require a large broad-wavelength decrease in the DC reflectance. We instead observe during these events that the DC increases at short wavelengths while the DC decreases at long wavelengths, suggesting primarily an exchange between HbO2 and HbR volume fractions, as opposed to a dramatic HbT change.
Regions A–C (Figures 12.7–12.9) were chosen to highlight three different time signatures observed in the field of view during the CSD dynamics. The most contrasting feature between all three regions is the measured AC reflectance and the derived scattering coefficient. In Region B (Figure 12.8), each CSD event appears to cause a biphasic scattering change, with a sharp increase and then decrease, whereas a monophasic dip was observed in Region A (Figure 12.7). Region C (Figure 12.9) appears even more complex with a triphasic rise-dip-rise temporal profile. We observe that Regions A to C are located with increasing proximity to the CSD induction point (3 mm above the imaging field).
Because fractional changes in scattering and absorption have an equal (and opposite) effect on DC reflectance (see Section 12.2.3.3), any scattering (i.e., pathlength) changes measured here could be misinterpreted as absorption events with traditional ISOI analyses (i.e., DC reflectance only). In our observations, the measured scattering change of up to −0.05 mm−1 would be interpreted as an increase in absorption of up to +0.005 mm−1, more than the maximum measured absorption change for wavelengths 730, 780, or 830 nm in any of the three regions. In order to account for differential pathlength changes, Kohl et al. proposed a multispectral model [39], which they used to differentiate dynamic scattering and absorption changes using ISOI. This approach improves ISOI accuracy, and has been generally adopted as the method of choice for quantitative functional imaging. For dynamic measurements, we see MI as an improvement over this approach as it alternatively uses frequency domain measurements at a single wavelength to derive absolute scattering and absorption coefficients. This potentially provides a simplified single-wavelength measurement apparatus for detection of scattering, and also avoids potential mis-estimation of background optical properties.
Light scattering changes induced by spreading depression have been reported previously, and a comprehensive review is provided by Somjen. With in vivo spatially resolved reflectance measurements, Kohl et al. [11] separated absorption from scattering and observed a biphasic scattering response similar to that of Region A. With simultaneous laser scattering and electrophysiological measurements, both Jarvis et al. and Tao et al. found a strong correlation between electrical and optical scattering changes [12,13,40]. Tao et al. noted spatial heterogeneity in the dynamic spreading depression (SD) waveform related to the proximity to the SD induction site, similar to our results.
Using linear spectral analysis of absorption at all four wavelengths, we calculated the time-dependent chromophore concentration for Regions A, B, and C, presented in Figure 12.10A,B,C, respectively. In each region, the calculated baseline concentrations of H2O were assumed to be constant. All three regions exhibit remarkably similar trends in HbR, HbO2, HbT, and StO2. This similarity is not clear in the DC traces of Figures 12.7–12.9, further highlighting the benefit of accurate separation of μa and μs′. Focusing on the first CSD event, there is a very consistent signature of: (1) a 2-minute latency post-KCl administration, (2) a 30-second period of decreasing StO2 (3) a dramatic spike in both StO2 (3–10%) and HbT (2–4 μM) with rise and decay times of approximately 1 minute each. For each region, the final StO2 is approximately 5–10% lower than baseline, while the HbT restores to baseline values. This process repeats again twice more, except that the phase (2) desaturation appears to be absent. Additionally, in the “vessel” Region 3, we observe a gradual increase in HbT over the 30 minutes, indicating chronic blood pooling.
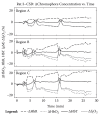
FIGURE 12.10
Recovered HbR, HbO2, HbT, and STO2, for ROIs A, B, and C (top, middle, and bottom), recovered by analysis of the multispectral absorption coefficients from Figures 12.7–12.9b (top).
We show in Figure 12.11 the spatio-temporal evolution of both chromophore concentration and scattering changes from the first SD wave in rat 3. These are depicted in the form of a time derivative, i.e., (C(tn + 1) − C(tn))/(tn + 1 − tn), where C represents concentration/saturation/scattering values and tn represents time of acquisition for data point n. This visualization is appealing as it highlights the changes with high contrast [18]. From left to right, we show HbO2, HbR, HbT, StO2, and μs′. Notice the wave in scattering which propagates from top right to bottom left, at a rate of approximately 3 mm/min. An increase, or “spike” in scattering is observed initially in the top right hand corner, in close proximity to the location of KCl administration. Note the large spikes in HbT and StO2 due to vascular activity from depression wave propagation through the measurement field. We observe a transient increase in saturation and blood volume. Over the longer time periods, however, we observe a slow, sustained trend toward hypoxia in the vein regions.

FIGURE 12.11
Spatio-temporal evolution of the hemodynamic and neural scattering response during a single spontaneous CSD event in rat 3. For visualization, a time derivative of the image sequence is displayed to highlight changes.
The spatio-temporal evolution of the scattering coefficient in Figure 12.11 reveals a spatially defined scattering wave (reduction in μs′) that precedes hemodynamic changes. The scattering drop is presumed to be a consequence of neuronal depolarization accompanying CSD. This observed wave pattern has been shown previously with reflectance ISOI and attributed to blood volume changes [18]. Interestingly, the scattering depolarization wave is clearly followed in space and time by the increase in deoxyhemoglobin (HbR), decrease in saturation (StO2), and drop in oxyhemoglobin (HbO2); changes that are consistent with depolarization-induced neural tissue oxygen consumption.
12.3.3. Dynamic MI Spectroscopy of Stroke
In order to assess the sensitivity of MI to stroke, we conducted preliminary studies in a rat middle cerebral artery occlusion (MCAo) model, the most commonly involved artery in ischemic strokes. The left MCA was surgically cauterized using monopolar cautery or ligated to produce a permanent stroke. Figure 12.12 shows pre-versus post-MCAo results for a representative animal. Data were acquired at 5 wavelengt
Original Source: https://www.ncbi.nlm.nih.gov/books/NBK20233/
Low-Level Laser Therapy in the Management of Mucositis and Dermatitis Induced by Cancer Therapy.
Bensadoun RJ1, Nair RG2,3. - Photomed Laser Surg. 2015 Oct;33(10):487-91. doi: 10.1089/pho.2015.4022. (Publication) 1
This great article is available for purchase.
View Resource

Abstract: PMID: 26447605 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26447605
Correction: Human Tubal-Derived Mesenchymal Stromal Cells Associated with Low Level Laser Therapy Significantly Reduces Cigarette Smoke-Induced COPD in C57BL/6 mice.
Peron JP, de Brito AA, Pelatti M, Brandão WN, Vitoretti LB, Greiffo FR, da Silveira EC, Oliveira-Junior MC, Maluf M, Evangelista L, Halpern S, Nisenbaum MG, Perin P, Czeresnia CE, Câmara NO, Aimbire F, de Paula Vieira R, Zatz M, de Oliveira AP. - PLoS One. 2015 Sep 25;10(9):e0139294. doi: 10.1371/journal.pone.0139294. () 12
View Resource
Background: [This corrects the article DOI: 10.1371/journal.pone.0136942.].
Abstract: PMID: 26406994 [PubMed - as supplied by publisher] PMCID: PMC4583288 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26406994
Histologic and Resonance Frequency Analysis of Peri-Implant Bone Healing After Low-Level Laser Therapy: An In Vivo Study.
Mayer L, Gomes FV, Carlsson L, Gerhardt-Oliveira M. - Int J Oral Maxillofac Implants. 2015 Sep-Oct;30(5):1028-35. doi: 10.11607/jomi.3382. () 16
View Resource
Background: To evaluate the effects of low-level laser therapy (LLLT) on peri-implant bone regeneration by means of resonance frequency analysis and histologic analysis of bone-to-implant contact (BIC).
Abstract: PMID: 26394337 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Thirty-two male New Zealand rabbits were randomly divided into four groups of eight animals each, one control group (nonirradiated animals) and three experimental groups that received LLLT (group E5 = 5 J per session; group E10 = 10 J per session; group E20 = 20 J per session). The mandibular left incisor was surgically extracted in all animals, and a nanoparticle-treated-surface osseointegrated implant was placed immediately afterward. The experimental groups were irradiated with aluminum-gallium-arsenide laser diode every 48 hours over a 13-day period for a total of seven sessions. Implant stability quotients (ISQs) were measured at the time of implant placement and 30 days after the last LLLT session. The animals were then euthanized and dissected, and histologic slides of the implant region were obtained for BIC evaluation.
Results: Significant differences in ISQ were detected between groups before and after LLLT, with group E20 showing significantly higher values than controls. The percentage of BIC was also significantly higher in group E20 than in control animals.
Conclusions: Laser therapy at a dose of 20 J per treatment session, based on the irradiation protocol used in this study, was able to significantly increase ISQ values and BIC after implant placement, indicating that laser irradiation effected an improvement in peri-implant bone healing.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26394337
Effect of low-level laser therapy (904 nm) and static stretching in patients with knee osteoarthritis: a protocol of randomised controlled trial.
Ferreira de Meneses SR1,2, Hunter DJ3, Young Docko E4, Pasqual Marques A5. - BMC Musculoskelet Disord. 2015 Sep 14;16:252. doi: 10.1186/s12891-015-0709-9. () 20
View Resource
Intro: Osteoarthritis (OA) is a highly prevalent and disabling disease. It is estimated that by 2030 the prevalence of symptomatic OA could reach 30 % of the population above 60 years. This randomised controlled trial will investigate the effect of low-level laser therapy (LLLT) and static stretching exercises, as monotherapy and in combination, on pain, quality of life, function, mobility, knee range of motion (KROM) and hamstring shortening in participants with knee OA.
Background: Osteoarthritis (OA) is a highly prevalent and disabling disease. It is estimated that by 2030 the prevalence of symptomatic OA could reach 30 % of the population above 60 years. This randomised controlled trial will investigate the effect of low-level laser therapy (LLLT) and static stretching exercises, as monotherapy and in combination, on pain, quality of life, function, mobility, knee range of motion (KROM) and hamstring shortening in participants with knee OA.
Abstract: Abstract
BACKGROUND:
Osteoarthritis (OA) is a highly prevalent and disabling disease. It is estimated that by 2030 the prevalence of symptomatic OA could reach 30 % of the population above 60 years. This randomised controlled trial will investigate the effect of low-level laser therapy (LLLT) and static stretching exercises, as monotherapy and in combination, on pain, quality of life, function, mobility, knee range of motion (KROM) and hamstring shortening in participants with knee OA.
METHODS:
This study will involve 145 people aged 50-75 years with symptomatic-radiographic knee OA. It will consist of two types of treatments: Low-level laser therapy (LLLT) and stretching exercises. The patients will be randomly allocated to five groups LLLTACTIVE+Stretch, LLLTPLACEBO+Stretch, Stretch, LLLT and Control (n = 29 each). Treatment frequency will be three sessions/week for all active groups. LLLT will involve the use of a Gallium-Arsenide laser (904 nm, 40 milliwatts, 3 J/point, 27 J/knee) over 24 sessions for the monotherapy group and 9 sessions for the LLLT+Stretch groups. Stretching will consist of seven exercises completed over 24 sessions. The control group will receive a booklet. Participants will be treated for 2 months (Stretch, LLLT and Control groups) or 3 months (LLLT + Stretch groups). Participants and the outcome assessor will be blind to treatment allocation throughout the study. The primary outcome is pain measured by Visual Analogue Scale. Secondary outcomes include quality of life assessed by Western Ontario and McMaster Universities Arthritis Index, function by Lequesne Algofunctional Index, mobility by Timed Up and Go Test, KROM by goniometry of knee flexion and hamstring shortening by popliteal angle. The statistical method will follow the principles of per-protocol analysis.
DISCUSSION:
Although exercise therapy is considered an effective treatment in patients with knee osteoarthritis, the knowledge of which exercise modalities would be the most appropriate for this population is lacking. LLLT has been used as resource to increase the effects of physical therapy. However, the specific dose and treatment frequency need to be better defined. The findings from this randomised controlled trial will provide evidence of the efficacy or otherwise, of LLLT and stretching exercises in the management of knee OA symptoms.
TRIAL REGISTRATION:
NCT01738737 at ClinicalTrials.gov.
Methods: This study will involve 145 people aged 50-75 years with symptomatic-radiographic knee OA. It will consist of two types of treatments: Low-level laser therapy (LLLT) and stretching exercises. The patients will be randomly allocated to five groups LLLTACTIVE+Stretch, LLLTPLACEBO+Stretch, Stretch, LLLT and Control (n = 29 each). Treatment frequency will be three sessions/week for all active groups. LLLT will involve the use of a Gallium-Arsenide laser (904 nm, 40 milliwatts, 3 J/point, 27 J/knee) over 24 sessions for the monotherapy group and 9 sessions for the LLLT+Stretch groups. Stretching will consist of seven exercises completed over 24 sessions. The control group will receive a booklet. Participants will be treated for 2 months (Stretch, LLLT and Control groups) or 3 months (LLLT + Stretch groups). Participants and the outcome assessor will be blind to treatment allocation throughout the study. The primary outcome is pain measured by Visual Analogue Scale. Secondary outcomes include quality of life assessed by Western Ontario and McMaster Universities Arthritis Index, function by Lequesne Algofunctional Index, mobility by Timed Up and Go Test, KROM by goniometry of knee flexion and hamstring shortening by popliteal angle. The statistical method will follow the principles of per-protocol analysis.
Results: Although exercise therapy is considered an effective treatment in patients with knee osteoarthritis, the knowledge of which exercise modalities would be the most appropriate for this population is lacking. LLLT has been used as resource to increase the effects of physical therapy. However, the specific dose and treatment frequency need to be better defined. The findings from this randomised controlled trial will provide evidence of the efficacy or otherwise, of LLLT and stretching exercises in the management of knee OA symptoms.
Conclusions: NCT01738737 at ClinicalTrials.gov.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26369333
Efficacy of low-level laser therapy for the treatment of burning mouth syndrome: a randomized, controlled trial.
Spanemberg JC1, López JL2, de Figueiredo MA1, Cherubini K1, Salum FG1. - J Biomed Opt. 2015 Sep 1;20(9):98001. doi: 10.1117/1.JBO.20.9.098001. () 21
View Resource
Abstract: PMID: 26359814 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26359814
Efficacy of the long-pulsed 1064-nm neodymium:yttrium-aluminum-garnet laser (LPND) (rejuvenation mode) in the treatment of papulopustular rosacea (PPR): A pilot study of clinical outcomes and patient satisfaction in 30 cases.
Lee JH1, Kim M1, Bae JM1, Cho BK1, Park HJ2. - J Am Acad Dermatol. 2015 Aug;73(2):333-6. doi: 10.1016/j.jaad.2015.05.030. (Publication) 48
Using a 1064 nm laser showed favorable results in treating rosacea.
View Resource
o the Editor: Papulopustular rosacea (PPR) is traditionally treated with systemic and topical antibiotics or retinoids. Owing to flare-ups after discontinuation of therapy combined with frequent side effects, such as gastrointestinal discomfort, photosensitivity, and teratogenicity, alternative treatments need to be developed.1 No ideal laser treatment for PPR currently exists, and studies on the rejuvenation mode of long-pulsed 1064-nm neodymium:yttrium-aluminum-garnet laser (LPND) for PPR are lacking. This prospective case series evaluated the efficacy of the rejuvenation mode of LPND treatment for PPR. This study was approved by the ethics committee of the Catholic Medical Center Office of Human Research Protection Program (SC13RESE0196).
Thirty Korean patients with PPR were recruited in the Dermatology Department of Yeouido St Mary's Hospital from 2010 to 2013. Exclusion criteria were as follows: any previous treatment with laser or light-based devices; topical treatments with corticosteroids, metronidazole, or calcineurin inhibitors; and systemic treatments with antibiotics or retinoids during the prior 3 months. The patients were divided into 2 groups: 22 patients with mild- to moderate-grade PPR, according to Investigator Global Assessment, treated with laser only (group A); and 8 patients with severe-grade PPR treated with laser and doxycycline 100 mg twice daily (group B). All 30 patients underwent 3 treatment sessions, each with a 4-week interval. Patients used a topical anesthetic cream applied 30 minutes before laser treatment. Patients received full-face LPND (GentleMax; Candela, Wayland, MA) treatments at 40 to 50 J/cm2, with a pulse duration of 50 milliseconds, and a 10-mm spot size with a dynamic cooling device (Cryogen; Candela). Throughout this study, patients were instructed to use a moisturizer and a broad-spectrum sunscreen with an SPF of 30 or higher, and to avoid known triggering factors for rosacea.
Treatment efficacy was assessed using the 4-point severity grading system for rosacea at each visit and 4 weeks after the last treatment through blinded photographic evaluation by 3 dermatologists.2 Patients also evaluated their own rosacea symptoms at each visit (Table I).
Table IDemographic and baseline clinical characteristics of 30 subjects with papulopustular rosacea
Characteristic
Group A (laser alone, n = 22)
Group B (laser + doxycycline, n = 8)
Age, y, mean (range)
42.77 (23-62)
43.13 (38-51)
Gender, n (%)
Female
17 (77.3)
7 (87.5)
Male
5 (22.7)
1 (12.5)
Fitzpatrick skin type, n (%)
IV
2 (9)
0
V
22 (91)
8 (100)
Aggravation factor, n (%)
Heat
18 (81.8)
8 (100)
Emotional change
13 (59.1)
8 (100)
Exercise or bathing
12 (54.5)
8 (100)
Alcohol
9 (40.9)
6 (27.3)
Others
20 (66.6)
9 (30)
Investigator Global Assessment of baseline, n (%)
0
Mild
4 (13.3)
0
Moderate
18 (60)
0
Results and patient data are listed in Table I and Fig 1. Use of the rejuvenation mode of LPND significantly improved all outcome measures, including decreased papule/pustule activity and improved nontransient erythema score compared with baseline (Fig 2). In addition to these end points, LPND also had beneficial effects on clearance of symptoms such as transient erythema, pruritus, burning, and dryness. Excellent to good overall improvement was seen in 77.3% (17 of 22) of patients in group A and 87.5% (7 of 8) of patients in group B. In recent years, LPND has been widely used for photorejuvenation inducing destruction of telangiectases and reduction of wrinkles by dermal collagen remodeling.3, 4 Furthermore, through follicular ablation and selective photothermolysis, LPND has been reported to be effective for inflammatory lesions.5 We postulate multiple mechanisms of action of the rejuvenation mode of LPND to improve PPR. All treatments were well tolerated. No patients experienced purpura, hyperpigmentation, hypopigmentation, edema, or scarring. The adverse effects were minimal, and included temporary erythema and immediate mild pain, not interfering with the daily activities of the patients.

Fig 1
The clinical assessments by investigators (A) and patients (B) scored on the National Rosacea Society Expert Committee 4-point rosacea severity grading system (0, absent; 1, mild; 2, moderate; 3, severe) before treatment and at 4 weeks after 3 treatment session with long-pulsed neodymium:yttrium-aluminum-garnet laser.
Limitations of the study were the small number of subjects, absence of a control group, and the short follow-up period.
In conclusion, this study showed that mild to severe PPR responded favorably to the rejuvenation mode of LPND treatment. Thus, we suggest that this therapy could be a potentially effective monotherapy for patients with mild to moderate PPR, or as a combination therapy for severe PPR.
Abstract: PMID: 26183984 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26183984
Effect of Low-Level Laser Therapy on Human Adipose-Derived Stem Cells: In Vitro and In Vivo Studies.
Min KH1, Byun JH, Heo CY, Kim EH, Choi HY, Pak CS. - Aesthetic Plast Surg. 2015 Oct;39(5):778-82. doi: 10.1007/s00266-015-0524-6. Epub 2015 Jul 17. () 49
View Resource
Intro: Low-level laser therapy (LLLT) continues to receive much attention in many clinical fields. Also, LLLT has been used to enhance the proliferation of various cell lines, including stem cells. This study investigated the effect of LLLT on human adipose-derived stem cells (ADSCs) through in vitro and in vivo studies.
Background: Low-level laser therapy (LLLT) continues to receive much attention in many clinical fields. Also, LLLT has been used to enhance the proliferation of various cell lines, including stem cells. This study investigated the effect of LLLT on human adipose-derived stem cells (ADSCs) through in vitro and in vivo studies.
Abstract: Abstract
BACKGROUND:
Low-level laser therapy (LLLT) continues to receive much attention in many clinical fields. Also, LLLT has been used to enhance the proliferation of various cell lines, including stem cells. This study investigated the effect of LLLT on human adipose-derived stem cells (ADSCs) through in vitro and in vivo studies.
METHODS:
Low-level laser irradiation of cultured ADSCs was performed using a 830 nm Ga-Al-As (gallium-aluminum-arsenide) laser. Then, proliferation of ADSCs was quantified by a cell counting kit-8. In the in vivo study, irradiated ADSCs or non-irradiated ADSCs were transplanted, and then, low-level laser irradiation of each rat was performed as per the protocol. Cell viability was quantified by immunofluorescent staining using the human mitochondria antibody.
RESULTS:
In the in vitro study, the laser-irradiated groups showed an increase in absorbance compared to the control group. Also, in the in vivo study, there was a significant increase in the number of human ADSCs in the laser-irradiated groups compared to the control group (p < 0.001).
CONCLUSIONS:
Our study showed that LLLT could enhance the proliferation and viability of ADSCs. The ADSCs enhanced by LLLT could be applied in various clinical fields. With the use of LLLT, the proliferation and viability of various cells can be enhanced, besides ADSCs.
NO LEVEL ASSIGNED:
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors http://www.springer.com/00266 .
Methods: Low-level laser irradiation of cultured ADSCs was performed using a 830 nm Ga-Al-As (gallium-aluminum-arsenide) laser. Then, proliferation of ADSCs was quantified by a cell counting kit-8. In the in vivo study, irradiated ADSCs or non-irradiated ADSCs were transplanted, and then, low-level laser irradiation of each rat was performed as per the protocol. Cell viability was quantified by immunofluorescent staining using the human mitochondria antibody.
Results: In the in vitro study, the laser-irradiated groups showed an increase in absorbance compared to the control group. Also, in the in vivo study, there was a significant increase in the number of human ADSCs in the laser-irradiated groups compared to the control group (p < 0.001).
Conclusions: Our study showed that LLLT could enhance the proliferation and viability of ADSCs. The ADSCs enhanced by LLLT could be applied in various clinical fields. With the use of LLLT, the proliferation and viability of various cells can be enhanced, besides ADSCs.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26183254
Influence of postoperative low-level laser therapy on the osseointegration of self-tapping implants in the posterior maxilla: a 6-week split-mouth clinical study.
Mandić B, Lazić Z, Marković A, Mandić B, Mandić M, Djinić A, Mili�ić B. - Vojnosanit Pregl. 2015 Mar;72(3):233-40. () 100
View Resource
Background: Low-level laser therapy (LLLT) has been proven to stimulate bone repair, affecting cellular proliferation, differentiation and adhesion, and has shown a potential to reduce the healing time following implant placement. The aim of this clinical study was to investigate the influence of postoperative LLLT osseointegration and early success of self-tapping implants placed into low-density bone.
Abstract: PMID: 25958474 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Following the split-mouth design, self-tapping implants n = 44) were inserted in the posterior maxilla of 12 patients. One jaw side randomly received LLLT (test group), while the other side was placebo (control group). For LLLT, a 637 nm gallium-aluminum-arsenide (GaAlAs) laser (Medicolaser 637, Technoline, Belgrade, Serbia) with an output power of 40 mW and continuous wave was used. Low-level laser treatment was performed immediately after the surgery and then repeated every day in the following 7 days. The total irradiation dose per treatment was 6.26 J/cm2 per implant. The study outcomes were: implant stability, alkaline-phosphatase (ALP) activity and early implant success rate. The follow-up took 6 weeks.
Results: Irradiated implants achieved a higher stability compared with controls during the entire follow-up and the difference reached significance in the 5th postoperative week (paired t-test, p = 0.030). The difference in ALP activity between the groups was insignificant in any observation point (paired t-test, p > 0.05). The early implant success rate was 100%, regardless of LLLT usage.
Conclusions: LLLT applied daily during the first postoperative week expressed no significant influence on the osseointegration of self-tapping implants placed into low density bone of the posterior maxilla. Placement of self-tapping macro-designed implants into low density bone could be a predictable therapeutic procedure with a high early success rate regardless of LLLT usage.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25958474
Low-level light/laser therapy versus photobiomodulation therapy.
Anders JJ1, Lanzafame RJ, Arany PR. - Photomed Laser Surg. 2015 Apr;33(4):183-4. doi: 10.1089/pho.2015.9848. () 122
View Resource
Abstract: PMID: 25844681 [PubMed - in process] PMCID: PMC4390214 [Available on 2016-04-01]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25844681
Reply to comments on: “Efficacy of low-level laser therapy in the management of orthodontic pain: a systematic review and meta-analysis�.
He W, Li C, Zou S. - Lasers Med Sci. 2015 Feb;30(2):941-2. () 127
View Resource
Abstract: PMID: 25806375 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25806375
Successful treatment of refractory Hailey-Hailey disease with a 595-nm pulsed dye laser: a series of 7 cases.
Hunt KM1, Jensen JD2, Walsh SB2, Helms ME3, Soong VY4, Jacobson ES3, Sami N2, Huang CC2, Theos A2, Northington ME2. - J Am Acad Dermatol. 2015 Apr;72(4):735-7. doi: 10.1016/j.jaad.2014.12.023. () 132
View Resource
Abstract: PMID: 25773417 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25773417
Efficacy of surgical and non-surgical interventions on accelerating orthodontic tooth movement: a systematic review.
Kalemaj Z, DebernardI CL, Buti J. - Eur J Oral Implantol. 2015 Spring;8(1):9-24. () 148
View Resource
Background: To conduct a systematic review of randomised controlled trials (RCTs) evaluating the effect of surgical and non-surgical procedures on the acceleration of orthodontic tooth movement (OTM) as an adjunct to orthodontic therapy (OT) in order to estimate the efficacy of these procedures and the benefit of their use in everyday orthodontic practice.
Abstract: PMID: 25738176 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Literature search was performed on PubMed, Scopus, Web of Science and Cochrane databases up to July 2014. Inclusion criteria were: (1) RCTs; (2) orthodontic therapy on permanent dentition; (3) application of adjunctive surgical or non-surgical procedures for accelerating OTM; (4) measurement of tooth movement. The primary outcome measure was tooth movement expressed as cumulative tooth movement (CTM), rate of tooth movement (RTM) or time of tooth movement (TTM). Pain and discomfort, periodontal health, anchorage loss, bone and root changes, and undesired tooth movement were evaluated as secondary outcomes.
Results: Literature research identified 184 studies. After screening of titles, abstracts and full-text studies, fifteen fulfilled the inclusion criteria and were included in this review. Six of the included studies investigated the effect of corticotomies, one of interseptal bone reduction, four of lowlevel laser therapy (LLLT), three of intraoral/extraoral devices releasing extracorporeal shock waves (ESWT), pulsed electromagnetic field (PEMF) and electrical current, respectively, and one of injected substances (relaxin) as an adjunct to OT. Three studies resulted of high methodological quality, six of medium, and six of low quality. Interseptal bone reduction was reported to increase RTM during the first 2 months (P = 0.002) and CTM at 3 months (P = 0.003). Studies investigating corticotomy reported significantly increased RTM (up to 2.3 times) during the first months after intervention, whereas results on TTM and CTM were quite controversial ranging from non-significant to highly significant (up to three times of TTM increase). The heterogeneity between studies investigating corticotomy could not allow for quantitative synthesis of the findings. Out of four studies investigating LLLT three reported positive effect on OT. Due to inadequate statistical analysis of data from original articles, results could not be summarised in meta-analyses. Effects of both electrical current devices and PEMF devices on CTM were reported to be larger on the experimental sides than on the control sides (P < 0.001). The other interventions were reported to be of no statistical or clinical relevance.
Conclusions: In the short term, corticotomy can accelerate OTM whereas long-term effects are questionable, thus no firm conclusions can be made on its efficacy and benefit of clinical use. There is some evidence that LLLT can slightly accelerate OTM but this result is not significant and the effect estimated is not clinically relevant. The very limited research-based evidence suggesting beneficial effects of interseptal bone reduction, electrical current and PEMF on OTM does not allow for solid conclusions. More high quality clinical research is required in order to estimate the efficacy of adjunctive interventions on accelerating OTM and their potential clinical use.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25738176
Effects of tissue water content on the propagation of laser light during low-level laser therapy.
Kim S, Shin S, Jeong S. - J Biomed Opt. 2015 May;20(5):051027. doi: 10.1117/1.JBO.20.5.051027. () 200
View Resource
Background: This work reports that the laser fluence rate inside porcine skin varied notably with the change of tissue water content under the same laser irradiation conditions. The laser fluence rate inside skin tissue samples with varying water content was measured using an optical fiber sensor, while the target was irradiated either by a low-level 635 or 830 nm laser (50 mW/cm2). It was demonstrated that the distribution of laser fluence rate inside the target is strongly affected by tissue water content and its profile is determined by the water content dependency of optical properties at the laser wavelength.
Abstract: PMID: 25611979 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25611979
Contradictory effects of hypercholesterolemia and diabetes mellitus on the progression of abdominal aortic aneurysm.
Gertz SD1, Gavish L1, Mintz Y2, Beeri R3, Rubinstein C4, Gavish LY1, Berlatzky Y4, Appelbaum L5, Gilon D3. - Am J Cardiol. 2015 Feb 1;115(3):399-401. doi: 10.1016/j.amjcard.2014.11.012. Epub 2014 Nov 13. () 231
View Resource
Abstract: PMID: 25499403 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25499403
This month's edition of the Journal begins with four fascinating studies.
Goldberg DJ1. - J Cosmet Laser Ther. 2014 Dec;16(6):263. doi: 10.3109/14764172.2014.980096. () 256
View Resource
Abstract: PMID: 25402725 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25402725
Low-level laser therapy for androgenic alopecia.
Tin SS1, Wiwanitkit V2. - Int J Trichology. 2014 Oct;6(4):189. doi: 10.4103/0974-7753.142892. () 272
View Resource
Abstract: PMID: 25368481 [PubMed] PMCID: PMC4212301 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25368481
Ablative fractional photothermolysis in the treatment of scar contractures of the wrists and forearms: a retrospective data analysis.
Perry A1, Elston J2, Reynolds H3, Hawley L3, Kroonen L2, Uebelhoer NS3, Shumaker PR3. - J Am Acad Dermatol. 2014 Oct;71(4):841-2. doi: 10.1016/j.jaad.2014.06.002. () 320
View Resource
Abstract: PMID: 25219714 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25219714
Is there a place for lasers in periodontal therapy?
Wadia R. - Prim Dent J. 2014 Aug;3(3):57-61. () 328
View Resource
Background: This article aims to provide an overview on the clinical applications of lasers in periodontics.
Abstract: PMID: 25198641 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25198641
Low-level laser/light therapy for androgenetic alopecia.
Gupta AK, Lyons DC, Abramovits W. - Skinmed. 2014 May-Jun;12(3):145-7. () 349
View Resource
Background: Androgenetic alopecia (AGA) is a persistent and pervasive condition that affects men worldwide. Some common treatment options for AGA include hair prosthetics, oral and topical medications, and surgical hair restoration (SHR). Pharmaceutical and SHR treatments are associated with limitations including adverse side effects and significant financial burden. Low-level laser or light (LLL) devices offer alternative treatment options that are not typically associated with adverse side effects or significant costs. There are clinic- and home-based LLL devices. One home-based laser comb device has set a standard for others; however, this device requires time devoted to carefully moving the comb through the hair to allow laser penetration to the scalp. A novel helmet-like LLL device for hair growth has proven effective in preliminary trials and allows for hands-free use. Regardless, there are few clinical trials that have been conducted regarding LLL devices for AGA and results are mixed. Further research is required to establish the true efficacy of these devices for hair growth in comparison to existing alternative therapies.
Abstract: PMID: 25134310 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25134310
Accelerating tooth movement.
Keim RG1. - J Clin Orthod. 2014 Apr;48(4):213-4. () 371
View Resource
Abstract: PMID: 25084500 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25084500
The necessity for increased attention to pulsed low-level laser therapy.
Bayat M1. - Photomed Laser Surg. 2014 Aug;32(8):427-8. doi: 10.1089/pho.2014.9858. Epub 2014 Jul 11. () 396
View Resource
Abstract: PMID: 25014468 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25014468
Simulation of laser propagation through a three-layer human skin model in the spectral range from 1000 to 1900 nm.
Nasouri B, Murphy TE, Berberoglu H. - J Biomed Opt. 2014;19(7):075003. doi: 10.1117/1.JBO.19.7.075003. () 400
View Resource
Background: For understanding the mechanisms of low-level laser/light therapy (LLLT), accurate knowledge of light interaction with tissue is necessary. We present a three-dimensional, multilayer reduced-variance Monte Carlo simulation tool for studying light penetration and absorption in human skin. Local profiles of light penetration and volumetric absorption were calculated for uniform as well as Gaussian profile beams with different spreads over the spectral range from 1000 to 1900 nm. The results showed that lasers within this wavelength range could be used to effectively and safely deliver energy to specific skin layers as well as achieve large penetration depths for treating deep tissues, without causing skin damage. In addition, by changing the beam profile from uniform to Gaussian, the local volumetric dosage could increase as much as three times for otherwise similar lasers. We expect that this tool along with the results presented will aid researchers in selecting wavelength and laser power in LLLT.
Abstract: PMID: 25003752 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25003752
Tendinitis, an open avenue for low-level laser therapy.
Lopes-Martins RA1. - Photomed Laser Surg. 2014 Jul;32(7):369-70. doi: 10.1089/pho.2014.9859. () 403
View Resource
Abstract: PMID: 24992270 [PubMed - in process]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24992270
Pemphigus vulgaris and laser therapy: crucial role of dentists.
Pavlić V, Aleksić VV, Zubović N, Veselinović V. - Med Pregl. 2014 Jan-Feb;67(1-2):38-42. () 411
View Resource
Background: Pemphigus vulgaris is a relatively rare, chronic, autoimmune vesiculobullous disorder characterized by formation of intraepithelial vesiculae and/or bullae in the skin and mucous membrane. Systemic steroids are considered to be the standard first-line therapy for pemphigus vulgaris. However, for patients unresponsive to standard therapy, the new treatment modalities are being sought. Low-level laser therapy has been accepted as an alternative or adjunctive treatment modality for many conditions in medicine and dentistry. Therefore, this study was aimed at presenting the effects of low-level laser therapy in the treatment of pemphigus vulgaris and to emphasize the crucial role of dentists in early recognition and diagnosis of pemphigus vulgaris.
Abstract: PMID: 24964567 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: The articles published until May 2013 were obtained from the Medline/PubMed online database, using following search terms and key words: "laser therapy" and "pemphigus vulgaris", "low-level laser irradiation" and "pemphigus vulgaris", "lasers" and "pemphigus vulgaris" and "pemphigus vulgaris".
Results: Low-level laser therapy could result in immediate and significant analgesia and improved wound healing within the observation period and follow-up. Furthermore, a decrease in patients' discomfort as well as the absence of recurrence of the pemphigus vulgaris lesions has been claimed.
Conclusions: Even though available literature suggests that low-level laser therapy can be efficiently used in treatment of oral pemphigus vulgaris, either independently or as a part of combined therapy approach, these results should be interpreted with caution since there are no solid evidence-based proofs to provide the guidelines for the treatment of pemphigus vulgaris with low-level laser therapy. Therefore, further long-term randomized controlled clinical studies are necessary in order to give any solid recommendations on the use of low-level laser therapy in the treatment of pemphigus vulgaris.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24964567
Does low-level laser therapy enhance the efficacy of intravenous regional anesthesia?
Nesioonpour S, Akhondzadeh R, Mokmeli S, Moosavi S, Mackie M, Naderan M. - Pain Res Manag. 2014 Nov-Dec;19(6):e154-8. Epub 2014 Jun 19. () 415
View Resource
Background: The use of intravenous regional anesthesia (IVRA) is limited by pain resulting from the application of tourniquets and postoperative pain.
Abstract: PMID: 24945286 [PubMed - indexed for MEDLINE] PMCID: PMC4273713 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Methods: To assess the efficacy of low-level laser therapy added to IVRA for improving pain related to surgical fixation of distal radius fractures.
Results: The present double-blinded, placebo-controlled, randomized clinical trial involved 48 patients who were undergoing surgical fixation of distal radius fractures. Participants were randomly assigned to either an intervention group (n=24), who received 808 nm laser irradiation as 4 J�point for 20 s over ipsilateral three nerve roots in the cervical region corresponding to C5-C8 vertebrae, and 808 nm laser irradiation as 0.1 J�cm2 for 5 min in a tangential scanning mode over the affected extremity; or a control group (n=24), who underwent the same protocol and timing of laser probe application with the laser switched off. Both groups received the same IVRA protocol using 2% lidocaine.
Conclusions: The mean visual analogue scale scores were significantly lower in the laser-assisted group than in the lidocaine-only group on all measurements during and after operation (P<0.05). The mean time to the first need for fentanyl administration during the operation was longer in the laser group (P=0.04). The total amount of fentanyl administered to patients was significantly lower in the laser-assisted group (P=0.003). The laser group needed significantly less pethidine for pain relief (P=0.001) and at a later time (P=0.002) compared with the lidocaine-only group. There was no difference between the groups in terms of mean arterial pressure and heart rate.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24945286
A review of laser applications in orthodontics.
Kang Y, Rabie AB, Wong RW. - Int J Orthod Milwaukee. 2014 Spring;25(1):47-56. () 446
View Resource
Background: Laser technique now is widely applied in orthodontic treatment and proved to have many benefits. Soft tissue lasers can be used to perform gingivectomy, frenectomy and surgical exposure of tooth with less bleeding and swelling, improved precision, reduced pain and less wound contraction. Other laser applications include enamel etching and bonding and bracket debonding. Lower level lasers have the potential effects of pain control and accelerating tooth movement. Clinicians must be aware of the safety issues and risks associated with laser and receive proper training before the laser treatment is started.
Abstract: PMID: 24812743 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24812743
Re: The effect of increased maximum power output on perioperative and early postoperative outcome in photoselective vaporization of the prostate.
Kaplan SA. - J Urol. 2014 Apr;191(4):1058. doi: 10.1016/j.juro.2014.01.021. Epub 2014 Jan 15. () 472
View Resource
Abstract: PMID: 24703139 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24703139
Effects of low-level light therapy on facial corticosteroid addiction dermatitis: a retrospective analysis of 170 Asian patients.
Luan Q, Liu L, Wei Q, Liu B1. - Indian J Dermatol Venereol Leprol. 2014 Mar-Apr;80(2):194. doi: 10.4103/0378-6323.129436. () 480
View Resource
Abstract: PMID: 24685879 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24685879
Efficacy of red and infrared lasers in treatment of temporomandibular disorders--a double-blind, randomized, parallel clinical trial.
Pereira TS, Flecha OD, Guimarães RC, de Oliveira D, Botelho AM, Ramos Glória JC, Aguiar Tavano KT. - Cranio. 2014 Jan;32(1):51-6. () 486
View Resource
Background: Low-level laser therapy has still not been well established, and it is important to define a standardized protocol for the treatment of temporomandibular disorders (TMDs) using low level laser. There is no consensus on controlled clinical trials concerning the best option for laser therapy with regard to wavelength. The aim of this study was to evaluate the efficacy of red and infrared laser therapy in patients with TMD, using a randomized parallel-group double-blind trial.
Abstract: PMID: 24660647 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Each hemiface of 19 subjects was randomized to receive intervention, in a total of 116 sensitive points. Pain was measured at baseline and time intervals of 24 hours, 30 days, 90 days, and 180 days after treatment. Irradiation of 4 J/cm2 in the temporomandibular joints and 8 J/cm(2) in the muscles was used in three sessions.
Results: Both treatments had statistically significant results (P<0.001); there was statistical difference between them at 180 days in favor of the infrared laser (P=0.039). There was improvement in 24 hours, which extended up to 180 days in both groups.
Conclusions: Both lasers are effective in the treatment and remission of TMD symptoms.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24660647
Low-level laser therapy for management of TMJ osteoarthritis.
Madani AS, Ahrari F, Nasiri F, Abtahi M, Tunér J. - Cranio. 2014 Jan;32(1):38-44. () 487
View Resource
Background: This study investigated the efficacy of low-level laser therapy (LLLT) for the management of temporomandibular joint (TMJ) osteoarthritis.
Abstract: PMID: 24660645 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: In a double-blind clinical trial, 20 patients with TMJ osteoarthritis were randomly divided into laser and placebo groups. The patients in the laser group received irradiation from an 810 nm low-level laser (Peak power 80 W, average power 50 mW, 1500 Hz, 1 micro s pulse width, 120 seconds, 6 J, 3.4 J/cm(2) per point), which was applied on four points around the TMJs and on painful muscles three times a week for 4 weeks. In the placebo group, the treatment was the same as that in the laser group, but with laser simulation. The patients were evaluated before laser therapy (T1), after 6 (T2) and 12 (T3) laser applications and 1 month after the last application (T4), and the amount of mouth opening and the pain intensity were recorded.
Results: No significant differences were found in mouth opening either between the study groups or between the different evaluation times in each group (P>0.05). There was no significant difference in pain symptoms of the masticatory muscles and TMJ between the laser and the placebo groups (P>0.05), but some significant within-group improvements were present for Visual Analogue Scale (VAS) scores of the body of the masseter and TMJ in both groups.
Conclusions: LLLT using the present laser parameters was no more effective than the placebo treatment for reducing pain and improving mouth opening in patients with TMJ osteoarthritis.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24660645
Scientific rigor and strategic vision are the key points for going forward in photomedicine.
Trelles MA1. - Photomed Laser Surg. 2014 Apr;32(4):185. doi: 10.1089/pho.2014.9863. Epub 2014 Mar 24. () 488
View Resource
Abstract: PMID: 24661051 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24661051
Studies related to energy based devices.
Goldberg DJ1. - J Cosmet Laser Ther. 2014 Apr;16(2):47. doi: 10.3109/14764172.2014.889500. () 506
View Resource
Abstract: PMID: 24625134 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24625134
Riehl melanosis treated successfully with Q-switch Nd:YAG laser.
Smucker JE, Kirby JS. - J Drugs Dermatol. 2014 Mar;13(3):356-8. () 521
View Resource
Background: Riehl melanosis is a rare cause of skin hyperpigmentation that typically occurs on the face and neck and is characterized by the rapid onset of gray-brown reticular pigmentation. It is theorized to be a pigmented contact dermatitis or a lichenoid immune reaction that may be caused by intrinsic as well as extrinsic factors. Treatment is challenging; laser and intense pulsed light (IPL) therapy is a common treatment for other pigmented skin conditions. IPL has been reported twice for the treatment of Riehl melanosis and we report a case of Riehl melanosis successfully treated with q-switched Nd:YAG after proving recalcitrant to IPL treatment.
Abstract: PMID: 24595582 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24595582
Postherpetic neuralgia: case study of class 4 laser therapy intervention.
Knapp DJ. - Clin J Pain. 2013 Oct;29(10):e6-9. doi: 10.1097/AJP.0b013e31828b8ef8. () 578
View Resource
Background: Postherpetic neuralgia (PHN) is a neuropathic sequelae in 8% to 27% of individuals with prior varicella zoster virus infection and herpes zoster resulting in retrograde demyelination, neurotoxic reactive oxygen species levels, and proinflammatory cytokine activation of microglia. Pain management strategies are well documented, but not always effective. Laser therapy has shown utility in nerve injury-related pain disorders and was considered a potentially efficacious intervention.
Abstract: PMID: 24384987 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Case report.
Results: Class 4 therapeutic laser treatment was applied with a dual wavelength GaAlAs (810 nm), GaAl (980 nm) laser, 2 to 4 W, 50% duty cycle, 10 Hz pulse active phase, 2.5 cm diameter aperture, scanning technique with skin contact, 10-minute treatment, 600 to 1200 J total, energy density of 3.5 to 7.1 J/cm average per session, and power density from 0.41 to 0.82 W/cm for 8 treatments. Outcome measures included the Neuropathy Pain Scale Questionnaire as the primary outcome measure, with the Numeric Pain Scale and total area of allodynia touch sensitivity as secondary outcome measurements.
Conclusions: The author reports a case of PHN of 15-year duration resistant to prior interventions. Weekly laser therapy treatment over 8 weeks resulted in reduced 0 to 10 Numeric Pain Scale score from 8 to 0, Neuropathy Pain Scale Questionnaire total score from 39 to 4, and allodynia over a 60 cm surface area of the upper trunk and posterior arm totally resolved, with resolution continued at 14-month follow-up.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24384987
Fractionated carbon dioxide laser as a novel, noninvasive treatment approach to burn scar-related nail dystrophy.
Krakowski AC1, Admani S, Shumaker PR, Uebelhoer NS. - Dermatol Surg. 2014 Mar;40(3):351-4. doi: 10.1111/dsu.12418. Epub 2013 Dec 26. () 581
View Resource
Abstract: PMID: 24372626 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24372626
Low level laser therapy (LLLT) in veterinary medicine.
Godine RL1. - Photomed Laser Surg. 2014 Jan;32(1):1-2. doi: 10.1089/pho.2013.9867. Epub 2013 Dec 20. () 589
View Resource
Abstract: PMID: 24359266 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24359266
LLLT in combination with non-surgical periodontal therapy in patients with gingival oral lichen planus: a pilot study.
Gambino A1, Cafaro A1, Arduino PG1, Conrotto D1, Broccoletti R1. - Ann Stomatol (Roma). 2013 Oct 24;4(Suppl 2):19. eCollection 2013. () 594
View Resource
Abstract: PMID: 24353781 [PubMed] PMCID: PMC3860251 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24353781
Low level laser therapy (LLLT) as adjuvant in the management of drug induced gingival hyperplasia: a case report.
Cafaro A1, Arduino PG1, Broccoletti R1, Romagnoli E2. - Ann Stomatol (Roma). 2013 Oct 24;4(Suppl 2):8-9. eCollection 2013. () 595
View Resource
Abstract: PMID: 24353764 [PubMed] PMCID: PMC3860244 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24353764
High-level Evidence Exists for Low-level Laser Therapy on Chemoradiotherapy-induced Oral Mucositis in Cancer Survivors.
Kumar SP1, Prasad K2, Shenoy K3, D'Souza M4, Kumar VK5. - Indian J Palliat Care. 2013 Sep;19(3):195-6. doi: 10.4103/0973-1075.121542. () 597
View Resource
Abstract: PMID: 24347912 [PubMed] PMCID: PMC3853400 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24347912
Fox-Fordyce disease treatment with fractional CO2 laser.
Ahmed Al-Qarqaz F1, Al-Shannag R. - Int J Dermatol. 2013 Dec;52(12):1571-2. doi: 10.1111/j.1365-4632.2011.05294.x. () 628
View Resource
Abstract: PMID: 24261728 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24261728
Improvement in lupus pernio with the successive use of pulsed dye laser and nonablative fractional resurfacing.
Emer J1, Uslu U, Waldorf H. - Dermatol Surg. 2014 Feb;40(2):201-2. doi: 10.1111/dsu.12376. Epub 2013 Nov 14. () 633
View Resource
Abstract: PMID: 24237486 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24237486
Pulsed dye laser for the treatment of acquired progressive lymphangioma.
Flores S1, Baum C, Tollefson M, Davis D. - Dermatol Surg. 2014 Feb;40(2):218-21. doi: 10.1111/dsu.12383. Epub 2013 Nov 14. () 634
View Resource
Abstract: PMID: 24237619 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24237619
Reply to comments on: "Efficacy of low-level laser therapy in the management of orthodontic pain: a systematic review and meta-analysis"
He W1, Li C, Zou S. - Lasers Med Sci. 2013 Nov 14. [Epub ahead of print] () 635
View Resource
Abstract: PMID: 24232862 [PubMed - as supplied by publisher]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24232862
ALSUntangled no. 23: the Rife machine and retroviruses.
ALSUntangled Group. - Amyotroph Lateral Scler Frontotemporal Degener. 2014 Mar;15(1-2):157-9. doi: 10.3109/21678421.2013.850802. Epub 2013 Nov 13. () 638
View Resource
Abstract: PMID: 24219300 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24219300
Effect of low-level laser therapy (λ660 nm) on angiogenesis in wound healing: a immunohistochemical study in a rodent model.
Colombo F, Neto Ade A, Sousa AP, Marchionni AM, Pinheiro AL, Reis SR. - Braz Dent J. 2013;24(4):308-12. doi: 10.1590/0103-6440201301867. () 652
View Resource
Background: The aim of the present investigation was to evaluate the angiogenesis on dorsal cutaneous wounds in a rodent model treated with λ660 nm laser light. New vessel formation is a multistep process involving vessel sprouting, endothelial cell migration, proliferation and tube formation. Although several in vivo studies have shown that laser phototherapy influences tissue repair, a fully understanding of angiogenesis mechanisms are not yet known. Twenty-four young adult male Wistar rats weighing between 200 and 250 g were used. Under general anesthesia, one excisional wound was created on the dorsum of each animal and they were randomly distributed into two groups: one control and one treated with laser (λ660 nm, 16 mW, 10 J/cm2). Each group was subdivided into three subgroups according to the animal death timing (2, 4 and 6 days). Laser irradiation started immediately after surgery and was repeated every other day during the experiment and marked with Sirius Red, specific for collagen, and immunomarked with anti-TGF-β and anti-von Willebrand factor. Marked sections underwent histological analysis by light microscopy and the mean area of the wound of each animal was calculated and analyzed by ANOVA and Tukey's test (α=0.05). Although at some death periods, collagen expression and number of blood vessels on irradiated animals were higher than in the control ones, no significant differences were found at any time in relation to TGF-β expression (p>0.05). It was concluded that laser treatment (λ660 nm) contributed to increase angiogenesis.
Abstract: PMID: 24173246 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24173246
Lung cancer stem cells and low-intensity laser irradiation: a potential future therapy?
Crous AM, Abrahamse H. - Stem Cell Res Ther. 2013;4(5):129. () 663
View Resource
Background: Lung cancer is notably a significant threat when considering worldwide cancer-related deaths. Despite significant advances in treatment modalities, death rates as a result of cancer relapse remain high. Relapse can occur as a result of metastasis. Cancer stem cells (CSCs) have been implicated as an important contributory factor in the development of metastasis. CSCs have the same characteristics as normal stem cells; that is, they can proliferate indefinitely and are capable of both self-renewal and differentiating into specialized cells. The molecular and cellular characteristics of stem cells and CSCs are coded for by cell-specific genes, which can be analyzed by using molecular assays setting the standard to work from. Low-intensity laser irradiation (LILI) has been applied in the treatment of numerous diseases and pathological conditions. LILI has been shown to stimulate proliferation of cells, capillary growth, and cellular metabolism as observed by adenosine triphosphate activation. It has been shown, by using different dosing levels of LILI, to either stimulate or inhibit cellular functions. One treatment strategy used on cancer cells is photodynamic therapy (PDT), in which cancer cells are treated with a photosensitizer (PS) in combination with laser irradiation. PSs are non-toxic by themselves but, with light activation, cause reactive oxygen species generation, which causes cancer cell death. Cell-specific PSs are being developed for future cancer treatment. In this review, we look at the potential effects of LILI and PDT on lung CSCs.
Abstract: PMID: 24153107 [PubMed - indexed for MEDLINE] PMCID: PMC3854767 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24153107
Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched Neodymium-Doped Yttrium Aluminum Garnet laser.
Kim HW1, Kim EJ, Park HC, Ko JY, Ro YS, Kim JE. - J Cosmet Laser Ther. 2014 Jun;16(3):147-8. doi: 10.3109/14764172.2013.854623. Epub 2013 Nov 18. () 676
View Resource
Abstract: PMID: 24131068 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24131068
A practical comparison of Copper Bromide Laser for the treatment of vascular lesions.
Lee S, Lee T, Kim H, Kim J, Eun H, Kim R. - Conf Proc IEEE Eng Med Biol Soc. 2013;2013:3765-8. doi: 10.1109/EMBC.2013.6610363. () 684
View Resource
Background: The recent rapid growth in demand for aesthetic non-invasive laser treatments such as unwanted skin rejuvenation, removal of age-related vascular blemishes has led to a boom in the medical devices to treat these conditions. Among diverse laser for skin treatment, copper bromide laser is a very effective, safe, and well tolerated treatment for facial telangiectasia at various energy levels and the most important thing of the copper bromide laser device is that the stability of the energy. However there is no evidence about effective copper bromide laser's energy level for the treatment of vascular lesions. We compared energy stability and treatment performance between two energy levels in 2 W and 8 W which commonly use in laser treatment for the vascular lesions. 8 W copper bromide laser was more stable compared than 2 W copper bromide laser. Also, 8 W copper bromide laser was effectively superior to 2 W copper bromide laser in treatment of vascular legion. Consequently, 8 W copper bromide laser treatment for vascular lesion might be more suitable than 2 W copper bromide laser.
Abstract: PMID: 24110550 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24110550
Minimizing skin cancer surgical scars using ablative fractional Er:YAG laser treatment.
Cohen JL. - J Drugs Dermatol. 2013 Oct;12(10):1171-3. () 697
View Resource
Background: Scars from skin cancer surgery on the face can be quite prominent and not easily obscured by makeup. This report evaluates the use of an ablative fractional Er:YAG laser device for minimizing or blending scar lines in two patients who underwent repair of skin cancer defects on the face.
Abstract: PMID: 24085055 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Two patients underwent surgery to remove facial skin cancer tumors. The resulting scars after reconstruction of these skin cancer defects on the left cheek (Case 1) and right cheek (Case 2) each received 3 treatments with a fractional ablative laser device (ProFractional-XC, Sciton, Inc., Palo Alto, CA). Treatments were spaced about 1 month apart. Topical anesthetic cream applied 1 hour before treatment minimized patient discomfort during the procedure. Treatment depths ranged from 150 to 200 microns, 2 passes were performed, and coverage per pass was typically 22% and then 11% in the coagulation mode. Results were evaluated by digital photography before the initial treatment, approximately 4-5 weeks after each of the 3 treatments, and at approximately 7 months after the surgical procedures.
Results: The fractional Er:YAG laser device significantly improved postsurgical scar lines in each patient without significant adverse effects. Prior to the laser sessions, these scars demonstrated hypopigmentation, hyperpigmentation, neovascularization, or diminished pore structures compared to the surrounding skin. These pigmentary, vascular or textural issues were all significantly improved by the fractional ablative Er:YAG laser.
Conclusions: The ablative fractional laser device of the present report safely minimizes and improves facial scars demonstrating not only textural alterations but also some pigmentary and vascular changes after reconstruction of skin cancer defects.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24085055
A persistent discoloration of the neck sparing the submental area.
Bahrani B1, Khachemoune A. - JAAPA. 2013 Aug;26(8):17-8. () 700
View Resource
Abstract: PMID: 24049935 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24049935
Influence of long-term water storage and thermocycling on shear bond strength of glass-ionomer cement to Er:YAG laser-prepared dentin.
Colucci V, de Araújo Loiola AB, da Motta DS, do Amaral FL, Pécora JD, Corona SA. - J Adhes Dent. 2014 Feb;16(1):35-9. doi: 10.3290/j.jad.a30539. () 720
View Resource
Background: To evaluate the influence of long-term water storage and thermocycling on the shear bond strength of a glass-ionomer cement to Er:YAG-irradiated and bur-prepared dentin.
Abstract: PMID: 24000332 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Fifteen bovine incisors were selected and the roots removed. Crowns were sectioned into four pieces, resulting in 60 samples that were individually embedded in polyester resin (n = 15) and ground to plane the enamel and expose the dentin. The bonding site was delimited and samples were randomly assigned according to the method of cavity preparation: Er:YAG laser (250 mJ/4 Hz) or high-speed handpiece (diamond bur #2096). Samples were fixed to a metallic device, where glass-ionomer cement (GIC) cylinders were prepared. Subsequently, they were subdivided according to the duration of water storage (WS) and number of thermocycles (TCs) - 24 h WS/no TCs and 6 months WS/12,000 TCs - and subjected to a shear bond strength test (500 N at 0.5 mm/min).
Results: The duration of water storage and number of thermocycles tested had no statistically significant effect on the shear bond strength to laser-irradiated dentin (p > 0.05). For bur-prepared substrate, the long-term degradation process promoted a decrease in shear bond strength values (p < 0.05).
Conclusions: Long-term water storage and thermocycling did not affect shear bond strength of glass-ionomer cement bonded to Er:YAG laser-prepared dentin.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24000332
The evolving paradigm for the treatment of diabetic macular edema.
Telander D1, Hunter A, Hariprasad SM. - Ophthalmic Surg Lasers Imaging Retina. 2013 Jul-Aug;44(4):324-8. doi: 10.3928/23258160-20130715-02. () 757
View Resource
Abstract: PMID: 23883266 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23883266
[ Steroids, cold, laser and pressure. What "flattens" hypertrophic scars].
[Article in German] - MMW Fortschr Med. 2013 Apr 4;155(6):25. () 824
View Resource
Abstract: PMID: 23700692 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23700692
Which therapy works for melasma in pigmented skin: lasers, peels, or triple combination creams?
Sardana K, Chugh S, Garg VK. - Indian J Dermatol Venereol Leprol. 2013 May-Jun;79(3):420-2. doi: 10.4103/0378-6323.110771. () 853
View Resource
Abstract: PMID: 23619448 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23619448
[Tattoo removal with laser (interview by Dr. Beate Schumacher)].
[Article in German] - MMW Fortschr Med. 2013 Jan 21;155(1):22-3. () 873
View Resource
Abstract: PMID: 23573711 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23573711
A split-face comparison of ablative fractional lasers (CO(2) and Er:YAG) in Asian patients; postprocedure erythema, pain and patient's satisfaction.
Jung KE, Jung KH, Park YM, Lee JY, Kim TY, Kim HO, Kim HS. - J Cosmet Laser Ther. 2013 Apr;15(2):70-3. doi: 10.3109/14764172.2012.759053. Epub 2013 Mar 6. () 910
View Resource
Background: Fractional photothermolysis has become popular in the recent years and is currently widely used for the treatment of scars and for photo-rejuvenation purposes. The fractional photothermolysis is to thermally alter a 'fraction' of the skin, leaving intervening areas of normal skin untouched, which rapidly repopulate the altered columns of tissue. Fractional photothermolysis is subdivided into non-ablative and ablative fractional resurfacing. Ablative fractional resurfacing uses fractional CO(2) or Er:YAG lasers to create deeper columns of thermal damage.Few studies have compared fractional CO(2) and Er:YAG lasers on scars and cutaneous photodamages by a split trial. In this pilot study, we have compared the effects, down time, postprocedure erythema, pain of CO(2) and Er:YAG fractional lasers using analysis of clinical photographs, dermoscopic findings and patient's rate of satisfaction.
Abstract: PMID: 23464882 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23464882
In reply to Olson.
de Castro G Jr, Snitcovsky IM. - Int J Radiat Oncol Biol Phys. 2013 Mar 15;85(4):895-6. doi: 10.1016/j.ijrobp.2012.08.029. () 922
View Resource
Abstract: PMID: 23452447 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23452447
The authors respond.
Tumilty S, McDonough S, Hurley-Osing DA, Baxter GD. - Arch Phys Med Rehabil. 2013 Feb;94(2):408. doi: 10.1016/j.apmr.2012.09.036. Epub 2013 Jan 24. () 955
View Resource
Abstract: PMID: 23351678 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23351678
Re: thulium laser versus holmium laser transurethral enucleation of the prostate: 18-month follow-up data of a single center.
Kaplan SA. - J Urol. 2013 Feb;189(2):615-6. doi: 10.1016/j.juro.2012.10.084. Epub 2012 Dec 20. () 979
View Resource
Abstract: PMID: 23312184 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23312184
Re: thulium:YAG VapoEnucleation of the prostate in large glands: a prospective comparison using 70- and 120-W 2-μm lasers.
Kaplan SA. - J Urol. 2013 Feb;189(2):614-5. doi: 10.1016/j.juro.2012.10.083. Epub 2012 Oct 29. () 981
View Resource
Abstract: PMID: 23312183 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23312183
[The effect of time parameters of laser radiation on the efficiency of photodynamic therapy].
[Article in Russian] - Med Tekh. 2012 Nov-Dec;(6):32-6. () 986
View Resource
Abstract: PMID: 23304989 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23304989
[Item 223--Cutaneous angiomas].
[Article in French] - Ann Dermatol Venereol. 2012 Oct;139(11 Suppl):A185-91. doi: 10.1016/j.annder.2012.06.019. Epub 2012 Jul 26. () 1024
View Resource
Abstract: PMID: 23176844 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23176844
Low-intensity laser radiation changes amino acid composition of wheat callus tissues.
Dudareva LV1, Shmakov VN, Sobenin AM, Rudikovskaya EG, Salyaev RK. - Dokl Biochem Biophys. 2012 Sep-Oct;446:260-2. doi: 10.1134/S160767291205016X. Epub 2012 Nov 7. () 1062
View Resource
Abstract: PMID: 23132724 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23132724
[The laser in dermatology].
[Article in German] - J Dtsch Dermatol Ges. 2012 Sep;10(9):605-6. () 1075
View Resource
Abstract: PMID: 23094278 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23094278
Low-level laser therapy of leg ulcer in sickle cell anemia.
Bonini-Domingos CR1, Valente FM. - Rev Bras Hematol Hemoter. 2012;34(1):65-6. doi: 10.5581/1516-8484.20120018. () 1096
View Resource
Abstract: PMID: 23049388 [PubMed] PMCID: PMC3459610 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23049388
Focus on: lasers.
Benjamin SD1. - Dent Today. 2012 Sep;31(9):23. () 1106
View Resource
Abstract: PMID: 23019847 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23019847
Efficacy of low-level laser therapy in the management of orthodontic pain: a systematic review and meta-analysis.
He WL1, Li CJ, Liu ZP, Sun JF, Hu ZA, Yin X, Zou SJ. - Lasers Med Sci. 2013 Nov;28(6):1581-9. doi: 10.1007/s10103-012-1196-y. Epub 2012 Sep 22. () 1115
View Resource
Intro: This review aimed to identify the efficacy of low-level laser therapy (LLLT) in the management of orthodontic pain. This systematic review and meta-analysis was carried out in accordance with Cochrane Handbook and the PRISMA statement. An extensive literature search for RCTs, quasi-RCTs, and CCTs was performed through CENTRAL, PubMed, Embase, Medline, CNKI, and CBM up to October 2011. Risk of bias assessment was performed via referring to the Cochrane tool for risk of bias assessment. Meta-analysis was implemented using Review Manager 5.1. As a result, four RCTs, two quasi-RCTs, and two CCTs were selected from 152 relevant studies, including 641 patients from six countries. The meta-analysis demonstrated that 24% risk of incidence of pain was reduced by LLLT (RR = 0.76, 95% CI range 0.63-0.92, P = 0.006). In addition, compared to the control group, LLLT brought forward "the most painful day" (MD = -0.42, 95% CI range -0.74- -0.10, P = 0.009). Furthermore, the LLLT group also implied a trend of earlier end of pain compared with the control group (MD = -1.37, 95% CI range -3.37-0.64, P = 0.18) and the pseudo-laser group (MD = -1.04, 95% CI range -4.22-2.15, P = 0.52). However, because of the methodological shortcomings and risk of bias of included trials, LLLT was proved with limited evidence in delaying pain onset and reducing pain intensity. In the future, larger and better-designed RCTs will be required to provide clearer recommendations.
Background: This review aimed to identify the efficacy of low-level laser therapy (LLLT) in the management of orthodontic pain. This systematic review and meta-analysis was carried out in accordance with Cochrane Handbook and the PRISMA statement. An extensive literature search for RCTs, quasi-RCTs, and CCTs was performed through CENTRAL, PubMed, Embase, Medline, CNKI, and CBM up to October 2011. Risk of bias assessment was performed via referring to the Cochrane tool for risk of bias assessment. Meta-analysis was implemented using Review Manager 5.1. As a result, four RCTs, two quasi-RCTs, and two CCTs were selected from 152 relevant studies, including 641 patients from six countries. The meta-analysis demonstrated that 24% risk of incidence of pain was reduced by LLLT (RR = 0.76, 95% CI range 0.63-0.92, P = 0.006). In addition, compared to the control group, LLLT brought forward "the most painful day" (MD = -0.42, 95% CI range -0.74- -0.10, P = 0.009). Furthermore, the LLLT group also implied a trend of earlier end of pain compared with the control group (MD = -1.37, 95% CI range -3.37-0.64, P = 0.18) and the pseudo-laser group (MD = -1.04, 95% CI range -4.22-2.15, P = 0.52). However, because of the methodological shortcomings and risk of bias of included trials, LLLT was proved with limited evidence in delaying pain onset and reducing pain intensity. In the future, larger and better-designed RCTs will be required to provide clearer recommendations.
Abstract: Abstract
This review aimed to identify the efficacy of low-level laser therapy (LLLT) in the management of orthodontic pain. This systematic review and meta-analysis was carried out in accordance with Cochrane Handbook and the PRISMA statement. An extensive literature search for RCTs, quasi-RCTs, and CCTs was performed through CENTRAL, PubMed, Embase, Medline, CNKI, and CBM up to October 2011. Risk of bias assessment was performed via referring to the Cochrane tool for risk of bias assessment. Meta-analysis was implemented using Review Manager 5.1. As a result, four RCTs, two quasi-RCTs, and two CCTs were selected from 152 relevant studies, including 641 patients from six countries. The meta-analysis demonstrated that 24% risk of incidence of pain was reduced by LLLT (RR = 0.76, 95% CI range 0.63-0.92, P = 0.006). In addition, compared to the control group, LLLT brought forward "the most painful day" (MD = -0.42, 95% CI range -0.74- -0.10, P = 0.009). Furthermore, the LLLT group also implied a trend of earlier end of pain compared with the control group (MD = -1.37, 95% CI range -3.37-0.64, P = 0.18) and the pseudo-laser group (MD = -1.04, 95% CI range -4.22-2.15, P = 0.52). However, because of the methodological shortcomings and risk of bias of included trials, LLLT was proved with limited evidence in delaying pain onset and reducing pain intensity. In the future, larger and better-designed RCTs will be required to provide clearer recommendations.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23001570
Ulceration of mature surgical scars following nonablative fractional photothermolysis associated with intralesional lidocaine injections.
Chuang GS1, Manstein D, Tannous Z, Avram MM. - Dermatol Surg. 2012 Nov;38(11):1879-81. doi: 10.1111/j.1524-4725.2012.02576.x. Epub 2012 Sep 13. () 1130
View Resource
Abstract: PMID: 22973818 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22973818
A rare case of congenital angiokeratoma of the glans penis treated using a 595-nm pulsed dye laser.
Burnett CT1, Kouba DJ. - Dermatol Surg. 2012 Dec;38(12):2028-30. doi: 10.1111/j.1524-4725.2012.02546.x. Epub 2012 Aug 3. () 1174
View Resource
Abstract: PMID: 22861497 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22861497
Questions regarding low-level laser therapy article.
Spielholz NI. - Am J Orthod Dentofacial Orthop. 2012 Jul;142(1):3. doi: 10.1016/j.ajodo.2012.05.007. () 1218
View Resource
Abstract: PMID: 22748981 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22748981
Low-level laser therapy for oral mucous membrane pemphigoid.
Cafaro A1, Broccoletti R, Arduino PG. - Lasers Med Sci. 2012 Nov;27(6):1247-50. doi: 10.1007/s10103-012-1137-9. Epub 2012 Jun 16. () 1238
View Resource
Abstract: PMID: 22706567 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22706567
Treatment of chemical leukoderma using a 308-nm excimer laser.
Ghazi E1, Ragi J, Milgraum S. - Dermatol Surg. 2012 Aug;38(8):1407-9. doi: 10.1111/j.1524-4725.2012.02443.x. Epub 2012 Jun 8. () 1241
View Resource
Abstract: PMID: 22681785 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22681785
A novel 0.65 millisecond pulsed 1064 nm laser to treat skin of color without skin cooling or anesthetics.
Cook-Bolden F1. - J Drugs Dermatol. 2011 Dec;10(12 Suppl):s10-1. () 1275
View Resource
Abstract: PMID: 22577684 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22577684
Letter regarding "management of ear lobule keloids using 980-nm diode laser".
Park TH, Chang CH. - Eur Arch Otorhinolaryngol. 2012 Aug;269(8):2017. doi: 10.1007/s00405-012-2013-8. Epub 2012 Apr 8. () 1313
View Resource
Abstract: PMID: 22484553 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22484553
Effect of 635nm Low-level Laser Therapy on Upper Arm Circumference Reduction: A Double-blind, Randomized, Sham-controlled Trial.
Nestor MS, Zarraga MB, Park H. - J Clin Aesthet Dermatol. 2012 Feb;5(2):42-8. () 1320
View Resource
Background: To assess the safety and efficacy of low-level laser therapy as a noninvasive method for reducing upper arm circumference.
Abstract: PMID: 22468172 [PubMed] PMCID: PMC3315881 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Methods: Randomized, double-blind study whereby healthy subjects (N=40) with a body mass index of 20 to 35kg/m(2) received three 20-minute low-level laser therapy (N=20) or sham treatments (N=20) each week for two weeks.
Results: Upper arm circumference was measured after three and six treatments and two weeks post-treatment. Primary success criterion was the proportion of subjects achieving a combined reduction in arm circumference of ≥1.25cm measured at three equally spaced points between the elbow and the shoulder. Secondary outcomes included total measurement change at each time point and subjective satisfaction ratings.
Conclusions: After six treatments, the low-level laser therapy group showed a combined reduction in arm circumference of 3.7cm versus 0.2cm in the sham treatment group (p<0.0001). Significantly more subjects in the low-level laser therapy group (N=12; 60%) achieved ≥1.5cm total decrease in upper arm circumference versus sham-treated subjects (N=0; 0%) (p<0.0005). Low-level laser therapy treatment resulted in a combined reduction in arm circumference of 2.2cm after three treatments and 3.7cm after six treatments (for each, p<0.0001) indicating a progressive and cumulative treatment effect. Body mass index remained unchanged for all subjects. A significantly greater number of subjects in the low-level laser therapy treatment group were satisfied with their results (p<0.05), believed their upper arm appearance improved (p<0.0005), and indicated the results exceeded expectations (p<0.05). The treatments were painless and no adverse events were reported.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22468172
LLLT in the Symptomatic Treatment of Oral Lichen Planus.
Fornaini C1. - Laser Ther. 2012 Mar 28;21(1):51-3. doi: 10.5978/islsm.12-CR-03. () 1325
View Resource
Abstract: PMID: 24610982 [PubMed] PMCID: PMC3944594 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24610982
On the edge.
Arnheiter H. - Pigment Cell Melanoma Res. 2012 Jan;25(1):1. doi: 10.1111/j.1755-148X.2011.00940.x. () 1422
View Resource
Abstract: PMID: 22176508 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22176508
[Experiences from general practice. 30 to 50% of patients have a good outcome with the laser (interview by Dr. Beate Schumacher)].
[Article in German] - MMW Fortschr Med. 2011 Nov 17;153(46):18. () 1427
View Resource
Abstract: PMID: 22145236 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22145236
[Cosmetic therapy of hirsutism. With laser and electric current against a hairy problem].
[Article in German] - MMW Fortschr Med. 2011 Nov 17;153(46):14-6. () 1429
View Resource
Abstract: PMID: 22145235 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22145235
Fractional laser resurfacing of acne scarring in patients with Fitzpatrick skin types IV-VI.
Alexis AF1. - J Drugs Dermatol. 2011 Dec;10(12 Suppl):s6-7. () 1433
View Resource
Abstract: PMID: 22134557 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22134557
Physiotherapy in hip and knee osteoarthritis: development of a practice guideline concerning initial assessment, treatment and evaluation.
Peter WF1, Jansen MJ, Hurkmans EJ, Bloo H, Dekker J, Dilling RG, Hilberdink W, Kersten-Smit C, de Rooij M, Veenhof C, Vermeulen HM, de Vos RJ, Schoones JW, Vliet Vlieland TP; Guideline Steering Committee - Hip and Knee Osteoarthritis. - Acta Reumatol Port. 2011 Jul-Sep;36(3):268-81. () 1440
View Resource
Intro: An update of a Dutch physiotherapy practice guideline in Hip and Knee Osteoarthritis (HKOA) was made, based on current evidence and best practice.
Background: An update of a Dutch physiotherapy practice guideline in Hip and Knee Osteoarthritis (HKOA) was made, based on current evidence and best practice.
Abstract: Abstract
BACKGROUND:
An update of a Dutch physiotherapy practice guideline in Hip and Knee Osteoarthritis (HKOA) was made, based on current evidence and best practice.
METHODS:
A guideline steering committee, comprising 10 expert physiotherapists, selected topics concerning the guideline chapters: initial assessment, treatment and evaluation. With respect to treatment a systematic literature search was performed using various databases, and the evidence was graded (1-4). For the initial assessment and evaluation mainly review papers and textbooks were used. Based on evidence and expert opinion, recommendations were formulated. A first draft of the guideline was reviewed by 17 experts from different professional backgrounds. A second draft was field-tested by 45 physiotherapists.
RESULTS:
In total 11 topics were selected. For the initial assessment, three recommendations were formulated, pertaining to history taking, red flags, and formulating treatment goals. Concerning treatment, 7 recommendations were formulated; (supervised) exercise therapy, education and self management interventions, a combination of exercise and manual therapy, postoperative exercise therapy and taping of the patella were recommended. Balneotherapy and hydrotherapy in HKOA, and thermotherapy, TENS, and Continuous Passive Motion in knee OA were neither recommended nor discouraged. Massage therapy, ultrasound, electrotherapy, electromagnetic field, Low Level Laser Therapy, preoperative physiotherapy and education could not be recommended. For the evaluation of treatment goals the following measurement instruments were recommended: Lequesne index, Western Ontario and McMaster Universities osteoarthritis index, Hip disability and Osteoarthritis Outcome Score and Knee injury and Osteoarthritis Outcome Score, 6-minute walktest, Timed Up and Go test, Patient Specific Complaint list, Visual Analoge Scale for pain, Intermittent and Constant OsteoArthritis Pain Questionnaire, goniometry, Medical Research Council for strength, handheld dynamometer.
CONCLUSIONS:
This update of a Dutch physiotherapy practice guideline on HKOA included 11 recommendations on the initial assessment, treatment and evaluation. The implementation of the guideline in clinical practice needs further evaluation.
Methods: A guideline steering committee, comprising 10 expert physiotherapists, selected topics concerning the guideline chapters: initial assessment, treatment and evaluation. With respect to treatment a systematic literature search was performed using various databases, and the evidence was graded (1-4). For the initial assessment and evaluation mainly review papers and textbooks were used. Based on evidence and expert opinion, recommendations were formulated. A first draft of the guideline was reviewed by 17 experts from different professional backgrounds. A second draft was field-tested by 45 physiotherapists.
Results: In total 11 topics were selected. For the initial assessment, three recommendations were formulated, pertaining to history taking, red flags, and formulating treatment goals. Concerning treatment, 7 recommendations were formulated; (supervised) exercise therapy, education and self management interventions, a combination of exercise and manual therapy, postoperative exercise therapy and taping of the patella were recommended. Balneotherapy and hydrotherapy in HKOA, and thermotherapy, TENS, and Continuous Passive Motion in knee OA were neither recommended nor discouraged. Massage therapy, ultrasound, electrotherapy, electromagnetic field, Low Level Laser Therapy, preoperative physiotherapy and education could not be recommended. For the evaluation of treatment goals the following measurement instruments were recommended: Lequesne index, Western Ontario and McMaster Universities osteoarthritis index, Hip disability and Osteoarthritis Outcome Score and Knee injury and Osteoarthritis Outcome Score, 6-minute walktest, Timed Up and Go test, Patient Specific Complaint list, Visual Analoge Scale for pain, Intermittent and Constant OsteoArthritis Pain Questionnaire, goniometry, Medical Research Council for strength, handheld dynamometer.
Conclusions: This update of a Dutch physiotherapy practice guideline on HKOA included 11 recommendations on the initial assessment, treatment and evaluation. The implementation of the guideline in clinical practice needs further evaluation.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22113602
A possible mechanism for treating staphylococcus aureus-induced chronic osteomyelitis in rats using 808-nm light.
Lubart R. - Photomed Laser Surg. 2011 Dec;29(12):789-90; author reply 791. doi: 10.1089/pho.2011.9898. Epub 2011 Nov 22. () 1444
View Resource
Abstract: PMID: 22107485 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22107485
Commentary on treatment of acne scars in Asian Patients using a 2,790-nm fractional yttrium scandium gallium garnet laser.
Perez M. - Dermatol Surg. 2011 Oct;37(10):1470-2. doi: 10.1111/j.1524-4725.2011.02116.x. () 1455
View Resource
Abstract: PMID: 22092942 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22092942
Prevention of recurrent herpes labialis outbreaks through low-intensity laser therapy: a clinical protocol with 3-year follow-up.
Eduardo Cde P1, Bezinelli LM, Eduardo Fde P, da Graça Lopes RM, Ramalho KM, Bello-Silva MS, Esteves-Oliveira M. - Lasers Med Sci. 2012 Sep;27(5):1077-83. doi: 10.1007/s10103-011-1019-6. Epub 2011 Nov 16. () 1459
View Resource
Abstract: PMID: 22086666 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22086666
Manual labor metacarpophalangeal arthropathy in a truck driver: a case report.
Emary PC. - J Chiropr Med. 2010 Dec;9(4):193-9. doi: 10.1016/j.jcm.2010.07.001. Epub 2010 Oct 8. () 1486
View Resource
Background: The purpose of this study is to present an unusual and rarely described case of occupational hand arthropathy involving the metacarpophalangeal (MCP) joints.
Abstract: PMID: 22027112 [PubMed] PMCID: PMC3206566 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Methods: A 62-year-old male truck driver (of 35 years) presented to a chiropractic clinic with pain and stiffness along the third metacarpal and MCP joint of the left hand. Examination revealed severe pain and limited flexion at the third MCP joint. Bilateral radiographs demonstrated severe osteoarthritis (OA) of this joint in the left (nondominant) hand and mild-to-moderate (asymptomatic) OA in the same joint on the right. Results of laboratory blood tests were unremarkable for metabolic, inflammatory, or infectious joint disease.
Results: The patient was diagnosed with bilateral, third MCP joint OA associated with manual labor. He was treated unsuccessfully with a short course of low-level laser therapy, MCP joint mobilization, and hand-stretching exercises. After 3½ years, the patient continues to work despite ongoing and worsening symptoms. Three serial left hand radiographs are presented, highlighting the progressive nature of this arthropathy.
Conclusions: The differential diagnosis in patients presenting with manual labor MCP joint OA should include hemochromatosis and calcium pyrophosphate dihydrate crystal deposition disease. Because of the increased risk of serious systemic disease, it is imperative that these latter 2 disorders are ruled out before the former is diagnosed.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22027112
Use of a fractional ablative 10.6-μm carbon dioxide laser in the treatment of a morphea-related contracture.
Kineston D1, Kwan JM, Uebelhoer NS, Shumaker PR. - Arch Dermatol. 2011 Oct;147(10):1148-50. doi: 10.1001/archdermatol.2011.247. () 1492
View Resource
Abstract: PMID: 22006130 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22006130
Complications in comparing lasers and LED. Comment on Esper MA, Nicolau RA, Arisawa EA (2011) The effect of two phototherapy protocols on pain control in orthodontic procedure--a preliminary clinical study. Lasers Med Sci 26:657-663.
Tunér J, Jenkins P. - Lasers Med Sci. 2012 Nov;27(6):1257-8. doi: 10.1007/s10103-011-1004-0. Epub 2011 Oct 14. () 1495
View Resource
Abstract: PMID: 21997801 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21997801
Photomedicine and LLLT Literature Watch.
Carroll JD1. - Photomed Laser Surg. 2011 Sep;29(9):653-4. doi: 10.1089/pho.2011.9902. () 1531
View Resource
Abstract: PMID: 21895530 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21895530
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2011 Aug;29(8):589. doi: 10.1089/pho.2011.9905. () 1572
View Resource
Abstract: PMID: 21801008 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21801008
Scleroderma and raynaud's phenomenon improve with high-peak power laser therapy: a case report.
St Surin-Lord S1, Obagi S. - Dermatol Surg. 2011 Oct;37(10):1531-5. doi: 10.1111/j.1524-4725.2011.02093.x. Epub 2011 Jul 25. () 1574
View Resource
Abstract: PMID: 21790846 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21790846
Narrowband ultraviolet B phototherapy and serum folic acid level.
Wiwanitkit V. - Lasers Med Sci. 2012 May;27(3):685. doi: 10.1007/s10103-011-0966-2. Epub 2011 Jul 23. () 1579
View Resource
Abstract: PMID: 21786023 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21786023
Pigmentary sequelae of AIDS-related cutaneous Kaposi sarcoma: successful treatment by Q-switched 755-nm alexandrite and 532-nm Nd:YAG lasers.
Hughes R1, Lacour JP, Passeron T. - Arch Dermatol. 2011 Jul;147(7):779-81. doi: 10.1001/archdermatol.2011.153. () 1583
View Resource
Abstract: PMID: 21768476 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21768476
[Laser treatment of trophic venous leg ulcers].
[Article in Russian] - Khirurgiia (Mosk). 2011;(4):64-7. () 1603
View Resource
Abstract: PMID: 21721284 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21721284
Granuloma faciale treated with 595-nm pulsed dye laser.
Hruza GJ, Ammirati CT. - Dermatol Surg. 2011 Jul;37(7):1060-1. doi: 10.1111/j.1524-4725.2011.02035.x. () 1607
View Resource
Abstract: PMID: 21711410 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21711410
Successful treatment of recurrent digital mucoid cysts using a 1,444-nm neodymium-doped yttrium aluminum garnet laser.
Kim JH1, Park JH, Jee H, Oh SH. - Dermatol Surg. 2011 Oct;37(10):1528-30. doi: 10.1111/j.1524-4725.2011.02085.x. Epub 2011 Jun 24. () 1611
View Resource
Abstract: PMID: 21707830 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21707830
Long-pulse neodymium-doped yttrium aluminum garnet laser treatment improves amiodarone-induced hyperpigmentation.
Bagheri S1, Eisen D. - Dermatol Surg. 2011 Oct;37(10):1539-41. doi: 10.1111/j.1524-4725.2011.02083.x. Epub 2011 Jun 24. () 1612
View Resource
Abstract: PMID: 21707831 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21707831
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2011 Jun;29(6):441-2. doi: 10.1089/pho.2011.9909. () 1632
View Resource
Abstract: PMID: 21631379 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21631379
Treatment of refractory discoid lupus erythematosus using 1,064-nm long-pulse neodymium-doped yttrium aluminum garnet laser.
Park KY1, Lee JW, Li K, Seo SJ, Hong CK. - Dermatol Surg. 2011 Jul;37(7):1055-6. doi: 10.1111/j.1524-4725.2011.02019.x. Epub 2011 May 25. () 1641
View Resource
Abstract: PMID: 21615600 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21615600
Treatment of bisphosphonate-induced osteonecrosis of the jaws with Nd:YAG laser biostimulation.
Luomanen M1, Alaluusua S. - Lasers Med Sci. 2012 Jan;27(1):251-5. doi: 10.1007/s10103-011-0929-7. Epub 2011 May 20. () 1650
View Resource
Abstract: PMID: 21597949 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21597949
[Principles of development of multifunctional equipment for low level laser and magnetolaser therapy].
[Article in Russian] - Med Tekh. 2011 Mar-Apr;(2):17-25. () 1655
View Resource
Abstract: PMID: 21574478 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21574478
[Is there any place for laser devices in the treatment of melasma?].
[Article in French] - Ann Dermatol Venereol. 2011;138(4):319-20. doi: 10.1016/j.annder.2010.12.016. Epub 2011 Feb 1. () 1672
View Resource
Abstract: PMID: 21497261 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21497261
Induction of de novo hair regeneration in scars after fractionated carbon dioxide laser therapy in three patients.
Beachkofsky TM1, Henning JS, Hivnor CM. - Dermatol Surg. 2011 Sep;37(9):1365-8. doi: 10.1111/j.1524-4725.2011.01934.x. Epub 2011 Apr 14. () 1675
View Resource
Abstract: PMID: 21492302 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21492302
Medical devices; general and plastic surgery devices; classification of the low level laser system for aesthetic use. Final rule.
Food and Drug Administration, HHS. - Fed Regist. 2011 Apr 14;76(72):20840-2. () 1677
View Resource
Background: The Food and Drug Administration (FDA) is classifying the low level laser system for aesthetic use into class II (special controls). The special control(s) that will apply to the device is entitled ``Class II Special Controls Guidance Document: Low Level Laser System for Aesthetic Use.'' The Agency is classifying the device into class II (special controls) in order to provide a reasonable assurance of safety and effectiveness of the device. Elsewhere in this issue of the Federal Register, FDA is announcing the availability of a guidance document that will serve as the special control for this device type.
Abstract: PMID: 21491809 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21491809
Is effect of low-level laser therapy in patients with burning mouth syndrome result of a placebo?
Vukoja D, Alajbeg I, Vu�ićević Boras V, Brailo V, Alajbeg IZ, Andabak Rogulj A. - Photomed Laser Surg. 2011 Sep;29(9):647-8; discussion 648, 651. doi: 10.1089/pho.2011.3005. Epub 2011 Apr 14. () 1678
View Resource
Abstract: PMID: 21492001 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21492001
Potassium titanyl phosphate 532-nm laser for treatment of a chronic nonhealing exophytic wound with hypergranulation tissue.
Madden K1, Paghdal KV, Cohen G. - Dermatol Surg. 2011 May;37(5):716-9. doi: 10.1111/j.1524-4725.2011.01976.x. Epub 2011 Apr 1. () 1701
View Resource
Abstract: PMID: 21457396 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21457396
About the treatment of xanthelasma palpebrarum using a 1,064 Q-switched neodymium-doped yttrium aluminum garnet laser.
Fusade T. - Dermatol Surg. 2011 Mar;37(3):403-4. doi: 10.1111/j.1524-4725.2011.01899.x. () 1720
View Resource
Abstract: PMID: 21410824 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21410824
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2011 Mar;29(3):213. doi: 10.1089/pho.2011.9916. () 1737
View Resource
Abstract: PMID: 21375456 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21375456
Immunohistochemical evaluation of skin before and after micro-ablative fractional erbium-doped yttrium aluminum garnet laser treatment.
Odo LM1, Odo ME, Guedes F, Sotto MN, Carlos Cucé L. - Dermatol Surg. 2011 Feb;37(2):246-8. doi: 10.1111/j.1524-4725.2010.01859.x. () 1754
View Resource
Abstract: PMID: 21324032 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21324032
Long-term follow-up of a case of cheek hyperpigmentation associated with McCune-Albright syndrome treated with Q-switched ruby laser.
Ozawa T1, Tateishi C, Shirakawa M, Murakami E, Ishii M, Harada T. - Dermatol Surg. 2011 Feb;37(2):263-6. doi: 10.1111/j.1524-4725.2010.01864.x. Epub 2011 Jan 27. () 1771
View Resource
Abstract: PMID: 21272121 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21272121
LLLT and PDT.
Lubart R1, Friedmann H. - Laser Ther. 2011;20(3):233. () 1808
View Resource
Abstract: PMID: 24155532 [PubMed] PMCID: PMC3799030 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24155532
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2010 Dec;28(6):849-50. doi: 10.1089/pho.2010.9923. () 1833
View Resource
Abstract: PMID: 21142728 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21142728
High-power diode laser use on Fordyce granule excision: a case report.
Baeder FM, Pelino JE, de Almeida ER, Duarte DA, Santos MT. - J Cosmet Dermatol. 2010 Dec;9(4):321-4. doi: 10.1111/j.1473-2165.2010.00531.x. () 1840
View Resource
Background: Fordyce granules are conventionally considered to be a developmental oral lesion with a higher incidence in men.
Abstract: PMID: 21122053 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: To report a clinical case of surgical lip Fordyce granule excision in a 19-year-old male.
Results: Fordyce granules were excised using a high-power diode laser (gallium arsenide [GaAs], Diode Vision®, MDL, 10 Dental Laser Unit, GmbH, Lower Saxony, Germany) with wavelength emission at 980 ± 10 nm, in a continuous wave mode, pulse width of 0.5 μs, fiber optic delivery system of 400 μm in diameter, at 2.5 W. Subsequently, low-intensity laser therapy was applied (gallium-aluminum-arsenide [GaAlAs], at 670 nm, 50 mW, at 4 J/cm(2); Dentoflex®, São Paulo, Brazil] in order to stimulate a faster wound tissue-healing process and less postoperative pain and inflammation.
Conclusions: The excellent esthetic result demonstrated the effectiveness of both high- and low-intensity laser therapy on the excision of Fordyce granules.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21122053
New insight into the pathophysiology of tattoo reactions following laser tattoo removal.
Harper J1, Losch AE, Otto SG, Zirwas M, Delaney KO, Wakelin JK 3rd. - Plast Reconstr Surg. 2010 Dec;126(6):313e-314e. doi: 10.1097/PRS.0b013e3181f63fde. () 1841
View Resource
Abstract: PMID: 21124109 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21124109
Inaccuracies in laser therapy meta-analysis for neck pain?
Bjordal JM, Lopes-Martins R, Johnson MI, Chow R. - J Physiother. 2010;56(4):282; author reply 283. () 1858
View Resource
Abstract: PMID: 21091418 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21091418
The successful treatment of Schamberg's disease with the 595 nm vascular laser.
D'Ambrosia RA1, Rajpara VS, Glogau RG. - Dermatol Surg. 2011 Jan;37(1):100-1. doi: 10.1111/j.1524-4725.2010.01822.x. Epub 2010 Nov 11. () 1868
View Resource
Abstract: PMID: 21070473 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21070473
Low-level laser therapy on hands of patients with rheumatoid arthritis.
Meireles SM, Jones A, Natour J. - Clin Rheumatol. 2011 Jan;30(1):147-8. doi: 10.1007/s10067-010-1601-5. Epub 2010 Oct 23. () 1896
View Resource
Abstract: PMID: 20972593 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20972593
[Oral venous malformation treated with pulsed-dye and neodymium:Yttrium-aluminum-garnet sequential laser].
[Article in Spanish] - Actas Dermosifiliogr. 2010 Oct;101(8):736-8. () 1900
View Resource
Abstract: PMID: 20965023 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20965023
Pulsed dye laser-resistant capillary malformation treated using intradermal sclerotherapy with ethanolamine oleate after intense pulsed light treatment.
Hwang SW1, Lee HJ, Hong SK, Seo JK, Lee D, Sung H. - Dermatol Surg. 2010 Nov;36(11):1775-8. doi: 10.1111/j.1524-4725.2010.01748.x. Epub 2010 Sep 24. () 1938
View Resource
Abstract: PMID: 20868377 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20868377
Disorders of tissue transformations of lysophosphatidylcholines at experimental pancreatic diabetes in white rats and peculiarities of the corrective effect of low-energy laser radiation of an extremely low intensity.
Burlakova EB1, Karagezyan KG, Amirkhanyan OM, Ovakimyan SS, Sekoyan ES. - Dokl Biochem Biophys. 2010 Jul-Aug;433:145-7. doi: 10.1134/S1607672910040010. Epub 2010 Aug 17. () 1965
View Resource
Abstract: PMID: 20714843 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20714843
Photomedicine and LLLT Literature Watch.
Carroll JD1. - Photomed Laser Surg. 2010 Aug;28(4):575-6. doi: 10.1089/pho.2010.9932. () 1969
View Resource
Abstract: PMID: 20704500 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20704500
Incorporating modalities into the rehab mix. Exploring pain solutions for athletes and weekend warriors.
O'Rourke J1. - Rehab Manag. 2010 Jul;23(6):14, 16-8. () 2038
View Resource
Abstract: PMID: 20614767 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20614767
Review conclusion for low-level laser therapy in shoulder impingement syndrome appears to be sensitive to alternative interpretations of trial results.
Bjordal JM. - J Rehabil Med. 2010 Jul;42(7):700-1; author reply 701-2. doi: 10.2340/16501977-0576. () 2040
View Resource
Abstract: PMID: 20603704 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20603704
Combination of Q-switched and quasi long-pulsed 1064-nm Nd:YAG laser, non-ablative 1450-nm diode laser, and ablative 10 600-nm carbon dioxide fractional laser for enlarged pores.
Cho SB, Noh S, Lee SJ, Kang JM, Kim YK, Lee JH. - J Dermatolog Treat. 2010 Jul;21(4):258-60. doi: 10.3109/09546630903410141. () 2060
View Resource
Background: Currently, there is no gold standard for the treatment of enlarged facial pores. In this report, we describe a patient with enlarged nasal pores which were treated with a combination of a non-ablative 1450-nm diode laser, a Q-switched and quasi long-pulsed 1064-nm Nd:YAG laser, and an ablative 10 600-nm carbon dioxide fractional laser system. Four months after the final treatment, the condition of the patient's pores had markedly improved, and the patient was satisfied with the results.
Abstract: PMID: 20509817 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20509817
Pulsed dye laser for port wine stains.
Cordoro KM, Frieden IJ. - J Am Acad Dermatol. 2010 Jun;62(6):1065-6. doi: 10.1016/j.jaad.2009.12.024. () 2075
View Resource
Abstract: PMID: 20466181 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20466181
Nevus comedonicus: a novel approach to treatment.
Givan J1, Hurley MY, Glaser DA. - Dermatol Surg. 2010 May;36(5):721-5. doi: 10.1111/j.1524-4725.2010.01537.x. Epub 2010 Apr 1. () 2114
View Resource
Abstract: PMID: 20384735 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20384735
Use of laser technology in orthodontics: hard and soft tissue laser treatments.
Genovese MD, Olivi G. - Eur J Paediatr Dent. 2010 Mar;11(1):44-8. () 2122
View Resource
Background: Modern technology has perfected a new instrument that has become almost indispensable in modern dentistry, in accordance with the philosophy of minimally invasive therapy: the laser. The aim of this work is to evaluate the effectiveness and efficacy of laser technology to solve mucogingival problems associated with orthodontic treatment. Some laser wavelengths work both on hard and soft tissues (2780 nm, 2940 nm), other lasers, such as the 810 nm diode, have a very good surgical and haemostatic action on soft tissues and an important analgesic and biostimulating effect that can help the healing of both TMJ painful symptoms as well as the pain following active orthodontic treatment. Several cases connected to orthodontic therapy are presented.
Abstract: PMID: 20359282 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: Different laser systems (diode laser at 810 nm; Er,Cr:YSGG laser at 2780 nm; Erbium:YAG laser at 2940 nm) were used, both for soft tissue surgery and enamel etching, and for biostimulating effect. These wavelengths were used with different parameters for each case, according to international current studies in view of minimally invasive therapy.
Results: The cases reported showed very quick and good healing of the laser treated tissues. These treatments, necessary for the orthodontic therapy or for its completion, become extremely simple, safe and rapid and the orthodontic specialist can perform them himself.
Conclusions: The laser technique is very effective in many operative and surgical procedures during orthodontic therapy. Further studies are however necessary to set the treatment protocols in orthodontic biostimulation.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20359282
Reply to: The use of low-level light for hair growth: Part I.
Stillman L. - J Cosmet Laser Ther. 2010 Apr;12(2):116. doi: 10.3109/14764171003674448. () 2133
View Resource
Abstract: PMID: 20331349 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20331349
Vitiligo therapy in children: a case for considering excimer laser treatment.
Patel N1, O'Haver J, Hansen RC. - Clin Pediatr (Phila). 2010 Sep;49(9):823-9. doi: 10.1177/0009922810363169. Epub 2010 Mar 22. () 2136
View Resource
Abstract: PMID: 20308196 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20308196
More experiments.
Ring D1. - J Hand Surg Am. 2010 Mar;35(3):473. doi: 10.1016/j.jhsa.2009.12.030. () 2148
View Resource
Abstract: PMID: 20193864 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20193864
Low-level laser treatment.
Bales JG1, Meals RA. - J Hand Surg Am. 2010 Mar;35(3):469-71; quiz 472. doi: 10.1016/j.jhsa.2009.12.029. () 2150
View Resource
Abstract: PMID: 20193863 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20193863
[Low level laser therapy: treatment option in neck pain].
[Article in German] - Dtsch Med Wochenschr. 2010 Feb;135(8):p7. doi: 10.1055/s-0030-1247671. Epub 2010 Feb 26. () 2151
View Resource
Abstract: PMID: 20191447 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20191447
Low-level laser therapy for neck pain.
Verhagen AP, Schellingerhout JM. - Lancet. 2010 Feb 27;375(9716):721; author reply 722. doi: 10.1016/S0140-6736(10)60296-6. () 2152
View Resource
Abstract: PMID: 20189016 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20189016
Low-level laser therapy for neck pain.
Shiri R, Viikari-Juntura E. - Lancet. 2010 Feb 27;375(9716):721-2; author reply 722. doi: 10.1016/S0140-6736(10)60297-8. () 2154
View Resource
Abstract: PMID: 20189015 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20189015
Fractional photothermolysis for the treatment of surgical scars.
Kunishige JH1, Katz TM, Goldberg LH, Friedman PM. - Dermatol Surg. 2010 Apr;36(4):538-41. doi: 10.1111/j.1524-4725.2010.01491.x. Epub 2010 Feb 19. () 2155
View Resource
Abstract: PMID: 20187893 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20187893
Removal of orange eyebrow tattoo in a single session with the Q-switched Nd:YAG 532-nm laser.
Guedes R1, Leite L. - Lasers Med Sci. 2010 May;25(3):465-6. doi: 10.1007/s10103-009-0748-2. Epub 2010 Feb 13. () 2172
View Resource
Abstract: PMID: 20155297 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20155297
Removal of orange eyebrow tattoo in a single session with the Q-switched Nd:YAG 532-nm laser.
Guedes R1, Leite L. - Lasers Med Sci. 2010 May;25(3):465-6. doi: 10.1007/s10103-009-0748-2. Epub 2010 Feb 13. () 2187
View Resource
Abstract: PMID: 20155297 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20155297
Basal cell nevus syndrome: a brave new world.
Goldberg LH1, Firoz BF, Weiss GJ, Blaydorn L, Jameson G, Von Hoff DD. - Arch Dermatol. 2010 Jan;146(1):17-9. doi: 10.1001/archdermatol.2009.322. () 2206
View Resource
Abstract: PMID: 20083687 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20083687
[CO(2)-laser in treatment of patients with basal cell face cancer].
[Article in Russian] - Stomatologiia (Mosk). 2009;88(5):74-5. () 2217
View Resource
Abstract: PMID: 20041520 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20041520
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2009 Dec;27(6):979-80. doi: 10.1089/pho.2009.9947. () 2221
View Resource
Abstract: PMID: 20035607 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20035607
Most important aspect of the treatment of severe burns is to close the wound as quickly as possible.
Pinheiro AL. - Photomed Laser Surg. 2009 Dec;27(6):965-6; author reply 967-8. doi: 10.1089/pho.2009.2667. () 2222
View Resource
Abstract: PMID: 20035605 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20035605
The diagnosis and treatment of peripheral lymphedema.
Piller N, Carati C. - Lymphology. 2009 Sep;42(3):146-7. () 2251
View Resource
Abstract: PMID: 19938271 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19938271
Current concepts review: noninsertional Achilles tendinopathy.
Courville XF1, Coe MP, Hecht PJ. - Foot Ankle Int. 2009 Nov;30(11):1132-42. doi: 10.3113/FAI.2009.1132. () 2268
View Resource
Abstract: PMID: 19912730 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19912730
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2009 Oct;27(5):829. doi: 10.1089/pho.2009.9948. () 2289
View Resource
Abstract: PMID: 19878032 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19878032
Fractional photothermolysis: a new therapeutic modality for xanthelasma.
Katz TM1, Goldberg LH, Friedman PM. - Arch Dermatol. 2009 Oct;145(10):1091-4. doi: 10.1001/archdermatol.2009.234. () 2313
View Resource
Abstract: PMID: 19841394 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19841394
Treatment for anogenital molluscum contagiosum.
Brown M1, Paulson C, Henry SL. - Am Fam Physician. 2009 Oct 15;80(8):864. () 2316
View Resource
Abstract: PMID: 19835348 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19835348
Treatment for anogenital molluscum contagiosum.
Brown M1, Paulson C, Henry SL. - Am Fam Physician. 2009 Oct 15;80(8):864. () 2328
View Resource
Abstract: PMID: 19835348 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19835348
Dermatosis papulosa nigra treatment with fractional photothermolysis.
Katz TM1, Goldberg LH, Friedman PM. - Dermatol Surg. 2009 Nov;35(11):1840-3. doi: 10.1111/j.1524-4725.2009.01302.x. Epub 2009 Oct 1. () 2345
View Resource
Abstract: PMID: 19796255 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19796255
[Laser therapy in oncological diseases and its hardware implementation].
[Article in Russian] - Med Tekh. 2009 Jul-Aug;(4):26-33. () 2352
View Resource
Abstract: PMID: 19777973 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19777973
Fractionated CO2 laser resurfacing: our experience with more than 2000 treatments.
Hunzeker CM, Weiss ET, Geronemus RG. - Aesthet Surg J. 2009 Jul-Aug;29(4):317-22. doi: 10.1016/j.asj.2009.05.004. () 2376
View Resource
Background: Fractionated carbon dioxide (CO(2)) laser resurfacing combines the concept of fractional photothermolysis with an ablative 10,600-nm wavelength. This technology allows for the effective treatment of rhytides, photodamage, and scars, with shorter recovery periods and a significantly reduced side effect profile as compared to traditional CO(2) laser resurfacing. In this article, the authors review the concept of fractional photothermolysis, the expanding array of indications for use of fractionated CO(2) lasers, and their preferred treatment technique.
Abstract: PMID: 19717066 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19717066
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2009 Aug;27(4):689-90. doi: 10.1089/pho.2009.9951. () 2396
View Resource
Abstract: PMID: 19694512 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19694512
Fractional photothermolysis for the treatment of postinflammatory hyperpigmentation.
Katz TM1, Goldberg LH, Firoz BF, Friedman PM. - Dermatol Surg. 2009 Nov;35(11):1844-8. doi: 10.1111/j.1524-4725.2009.01303.x. Epub 2009 Aug 13. () 2403
View Resource
Abstract: PMID: 19682001 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19682001
Treatment of angiofibromas of tuberous sclerosis with 5-aminolevulinic acid blue light photodynamic therapy followed by immediate pulsed dye laser.
Weinberger CH1, Endrizzi B, Hook KP, Lee PK. - Dermatol Surg. 2009 Nov;35(11):1849-51. doi: 10.1111/j.1524-4725.2009.01304.x. Epub 2009 Aug 13. () 2404
View Resource
Abstract: PMID: 19682000 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19682000
Successful treatment of severe keratosis pilaris rubra with a 595-nm pulsed dye laser.
Kaune KM1, Haas E, Emmert S, Schön MP, Zutt M. - Dermatol Surg. 2009 Oct;35(10):1592-5. doi: 10.1111/j.1524-4725.2009.01282.x. Epub 2009 Jul 28. () 2405
View Resource
Abstract: PMID: 19681988 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19681988
Lasers as aids for cleaning, shaping, and obturation of the root canal system.
Mohammadi Z1. - Dent Today. 2009 Jul;28(7):81-2, 84, 86; quiz 87, 80. () 2421
View Resource
Abstract: PMID: 19630279 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19630279
[Pathomorphological and histochemical manifestations of interaction of laser irradiation with tissues of a chorioretinal complex].
[Article in Russian] - Vestn Oftalmol. 2009 May-Jun;125(3):61-4. () 2440
View Resource
Abstract: PMID: 19566054 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19566054
Nonablative fractional photothermolysis for the treatment of striae rubra.
Katz TM1, Goldberg LH, Friedman PM. - Dermatol Surg. 2009 Sep;35(9):1430-3. doi: 10.1111/j.1524-4725.2009.01252.x. Epub 2009 Jun 22. () 2453
View Resource
Abstract: PMID: 19549177 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19549177
Role of artificial neural networks in dermatology.
Renders JM, Simonart T. - Dermatology. 2009;219(2):102-4. doi: 10.1159/000225933. Epub 2009 Jun 18. () 2458
View Resource
Abstract: PMID: 19546509 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19546509
Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report.
Roos S1, Raulin C, Ockenfels HM, Karsai S. - Dermatol Surg. 2009 Jul;35(7):1139-40. doi: 10.1111/j.1524-4725.2009.01202.x. Epub 2009 Apr 28. () 2499
View Resource
Abstract: PMID: 19438681 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19438681
Photomedicine and LLLT literature watch.
Carroll JD1. - Photomed Laser Surg. 2009 Apr;27(2):377-8. doi: 10.1089/pho.2009.9954. () 2516
View Resource
Abstract: PMID: 19382843 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19382843
[Effectiveness of extracorporal haemocorrection methods in the treatment of infectious endocarditis].
[Article in Russian] - Voen Med Zh. 2009 Feb;330(2):35-9. () 2525
View Resource
Abstract: PMID: 19351022 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19351022
Tissue-punch technique in nonattached tissue using the YSGG laser.
Kusek ER1. - Dent Today. 2009 Jan;28(1):132, 134-5. () 2541
View Resource
Abstract: PMID: 19323339 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19323339
[Laserotherapy and mesodiencephalic modulation after hemihepatoectomy in the early postoperative period].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2009 Jan-Feb;(1):39-40. () 2555
View Resource
Abstract: PMID: 19288600 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19288600
[The use of laseropuncture in patients with bronchial asthma and concomitant chronic rhinosinusitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2009 Jan-Feb;(1):37-9. () 2556
View Resource
Abstract: PMID: 19288599 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19288599
Is low-power pulsed laser ineffective in neural growth?
Tunér J. - Microsurgery. 2009;29(3):251. doi: 10.1002/micr.20616. () 2587
View Resource
Abstract: PMID: 19205062 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19205062
[Effectiveness of photo- and mesophotophoresis in the combined treatment of patients with chronic generalized parodontitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Nov-Dec;(6):42-3. () 2602
View Resource
Abstract: PMID: 19177664 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19177664
Permanent hyperpigmentation following laser hair removal using the dynamic cooling device.
Davidson D1, Ritacca D, Goldman MP. - J Drugs Dermatol. 2009 Jan;8(1):68-9. () 2603
View Resource
Abstract: PMID: 19180899 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19180899
[Chemotactic potential of phagocytes as a criterion for the efficacy of therapy in inflammatory diseases of the uterine appendages].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Nov-Dec;(6):41-2. () 2604
View Resource
Abstract: PMID: 19175057 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19175057
[Effectiveness of laser puncture in elderly patients with bronchial asthma].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Nov-Dec;(6):38-9. () 2606
View Resource
Abstract: PMID: 19175055 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19175055
Introduction. Update on lasers.
Uebelhoer NS1, Ross EV. - Semin Cutan Med Surg. 2008 Dec;27(4):221-6. doi: 10.1016/j.sder.2008.11.001. () 2616
View Resource
Abstract: PMID: 19150293 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19150293
Management of chronic pressure ulcers: an evidence-based analysis.
Health Quality Ontario. - Ont Health Technol Assess Ser. 2009;9(3):1-203. Epub 2009 Jul 1. () 2628
View Resource
Background: In April 2008, the Medical Advisory Secretariat began an evidence-based review of the literature concerning pressure ulcers.Please visit the Medical Advisory Secretariat Web site, http://www.health.gov.on.ca/english/providers/program/mas/tech/tech_mn.html to review these titles that are currently available within the Pressure Ulcers series.PRESSURE ULCER PREVENTION: an evidence based analysisThe cost-effectiveness of prevention strategies for pressure ulcers in long-term care homes in Ontario: projections of the Ontario Pressure Ulcer Model (field evaluation)MANAGEMENT OF CHRONIC PRESSURE ULCERS: an evidence-based analysis
Abstract: PMID: 23074533 [PubMed] PMCID: PMC3377577 Free PMC Article
Share on Facebook
Share on Twitter
Share on Google+
Methods: The Medical Advisory Secretariat (MAS) conducted a systematic review on interventions used to treat pressure ulcers in order to answer the following questions: Do currently available interventions for the treatment of pressure ulcers increase the healing rate of pressure ulcers compared with standard care, a placebo, or other similar interventions?Within each category of intervention, which one is most effective in promoting the healing of existing pressure ulcers?
Results: A pressure ulcer is a localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in conjunction with shear and/or friction. Many areas of the body, especially the sacrum and the heel, are prone to the development of pressure ulcers. People with impaired mobility (e.g., stroke or spinal cord injury patients) are most vulnerable to pressure ulcers. Other factors that predispose people to pressure ulcer formation are poor nutrition, poor sensation, urinary and fecal incontinence, and poor overall physical and mental health. The prevalence of pressure ulcers in Ontario has been estimated to range from a median of 22.1% in community settings to a median of 29.9% in nonacute care facilities. Pressure ulcers have been shown to increase the risk of mortality among geriatric patients by as much as 400%, to increase the frequency and duration of hospitalization, and to decrease the quality of life of affected patients. The cost of treating pressure ulcers has been estimated at approximately $9,000 (Cdn) per patient per month in the community setting. Considering the high prevalence of pressure ulcers in the Ontario health care system, the total cost of treating pressure ulcers is substantial.
Conclusions: Wounds normally heal in 3 phases (inflammatory phase, a proliferative phase of new tissue and matrix formation, and a remodelling phase). However, pressure ulcers often fail to progress past the inflammatory stage. Current practice for treating pressure ulcers includes treating the underlying causes, debridement to remove necrotic tissues and contaminated tissues, dressings to provide a moist wound environment and to manage exudates, devices and frequent turning of patients to provide pressure relief, topical applications of biologic agents, and nutritional support to correct nutritional deficiencies. A variety of adjunctive physical therapies are also in use.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23074533
[Experimental and clinical rationale for photophoresis therapy of chronic generalized parodontitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Sep-Oct;(5):42-3. () 2639
View Resource
Abstract: PMID: 19086104 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19086104
En coup de sabre presenting as a port-wine stain previously treated with pulsed dye laser.
Kakimoto CV1, Victor Ross E, Uebelhoer NS. - Dermatol Surg. 2009 Jan;35(1):165-7. doi: 10.1111/j.1524-4725.2008.34403.x. Epub 2008 Dec 6. () 2643
View Resource
Abstract: PMID: 19076183 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19076183
[Cellular mechanisms of magneto-laser treatment of reparative osteogenesis (an experimental study)].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Sep-Oct;(5):33-4. () 2644
View Resource
Abstract: PMID: 19069805 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19069805
[Laser therapy and "dry" carbonic acid gas baths in the combined treatment of patients with hypertensive disease and coronary heart disease].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2008 Sep-Oct;(5):3-5. () 2646
View Resource
Abstract: PMID: 19069795 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19069795
Excimer laser as adjuvant therapy for adult cutaneous Langerhans cell histiocytosis.
Vogel CA1, Aughenbaugh W, Sharata H. - Arch Dermatol. 2008 Oct;144(10):1287-90. doi: 10.1001/archderm.144.10.1287. () 2691
View Resource
Abstract: PMID: 18936391 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18936391
Facial scars after a road accident--combined treatment with pulsed dye laser and Q-switched Nd:YAG laser.
Martins A, Trindade F, Leite L. - J Cosmet Dermatol. 2008 Sep;7(3):227-9. doi: 10.1111/j.1473-2165.2008.00394.x. () 2719
View Resource
Background: We report the case of a woman who presented with several facial scars following a road accident. Treatment was carried out using combined laser treatment with pulsed dye laser (PDL) and Q-switched neodymium:yttrium-aluminum-garnet laser (QS Nd:YAG laser). No side effects or complications from treatment were noted or reported. The patient had very good cosmetic results with this combined technique. A variety of facial scars - erythematous, pigmented, atrophic, and hypertrophic - may occur as a result of trauma, surgery, burns, and skin disease. Surgery with other adjunctive methods including radiotherapy, intralesional steroids, and pressure therapy have shown variable results. Laser treatment has been attempted for scar revision since the 1980s. The PDL is the optimal treatment for reducing scar bulk and symptoms. It also decreases erythema and telangiectasia associated with scars, normalizes the skin surface texture, and improves the scar pliability. The QS Nd:YAG laser (1064 nm) is highly effective for traumatic tattoo removal, resulting in complete clearance in the majority of cases.
Abstract: PMID: 18789060 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18789060
Photomedicine and LLLT literature watch.
Carroll J1. - Photomed Laser Surg. 2008 Aug;26(4):409. doi: 10.1089/pho.2007.9972. () 2734
View Resource
Abstract: PMID: 18754723 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18754723
Re: Effect of low-level laser therapy on mast cells in second-degree burns in rats.
Aras MH. - Photomed Laser Surg. 2009 Feb;27(1):151; author reply 151. doi: 10.1089/pho.2008.2309. () 2757
View Resource
Abstract: PMID: 18687055 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18687055
Treatment of halo nevus with 308-nm excimer laser: a pilot study.
Bukhari IA. - J Cosmet Laser Ther. 2008 Dec;10(4):217. doi: 10.1080/14764170802123511. () 2782
View Resource
Abstract: PMID: 18618360 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18618360
Overviews and systematic reviews on low back pain.
Bjordal JM, Klovning A, Lopes-Martins RA, Roland PD, Joensen J, Slørdal L. - Ann Intern Med. 2008 May 20;148(10):789-90; author reply 791-2. () 2808
View Resource
Abstract: PMID: 18490694 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18490694
Nonablative fractional resurfacing for total body rejuvenation.
Narurkar VA1. - J Drugs Dermatol. 2008 Apr;7(4):352-5. () 2812
View Resource
Abstract: PMID: 18459516 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18459516
Blue foot: an unusual complication of tattoo and successful treatment with a QS-Nd:YAG laser.
Molenda MA1, Gehris RP, Jukic DM, Obagi S. - Dermatol Surg. 2008 Jul;34(7):947-9. doi: 10.1111/j.1524-4725.2008.34183.x. () 2835
View Resource
Abstract: PMID: 18384612 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18384612
Fractional photothermolysis.
Sukal SA1, Geronemus RG. - J Drugs Dermatol. 2008 Feb;7(2):118-22. () 2844
View Resource
Abstract: PMID: 18335647 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18335647
Hemangiomas in infancy and childhood. S 2k Guideline of the German Society of Dermatology with the working group Pediatric Dermatology together with the German Society for Pediatric Surgery and the German Society for Pediatric Medicine.
[Article in English, German] - J Dtsch Dermatol Ges. 2008 Apr;6(4):324-9. doi: 10.1111/j.1610-0387.2008.06657.x. Epub 2008 Feb 25. () 2853
View Resource
Abstract: PMID: 18312432 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18312432
[Using some new technologies in medical rehabilitation in chronic prostatitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2007 Nov-Dec;(6):34-42. () 2859
View Resource
Abstract: PMID: 18277406 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18277406
Photomedicine and LLLT literature watch.
Carroll J1. - Photomed Laser Surg. 2008 Feb;26(1):71-2. doi: 10.1089/pho.2007.9978. () 2866
View Resource
Abstract: PMID: 18248166 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18248166
Scar sarcoidosis in a child: case report of successful treatment with the pulsed dye laser.
Holzmann RD1, Astner S, Forschner T, Sterry G. - Dermatol Surg. 2008 Mar;34(3):393-6. doi: 10.1111/j.1524-4725.2007.34077.x. Epub 2008 Jan 7. () 2894
View Resource
Abstract: PMID: 18190544 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18190544
Lymphangioma circumscriptum: pitfalls and problems in definitive management.
Bond J1, Basheer MH, Gordon D. - Dermatol Surg. 2008 Feb;34(2):271-5. doi: 10.1111/j.1524-4725.2007.34054.x. Epub 2007 Dec 19. () 2896
View Resource
Abstract: PMID: 18177408 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18177408
Letter to the editor: inadequate statistical analysis hides significant effect of low level laser therapy in carpel tunnel syndrome.
Bjordal J. - Photomed Laser Surg. 2007 Dec;25(6):530-1. () 2906
View Resource
Abstract: PMID: 18158757 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18158757
Low-level laser therapy in oral and maxillofacial surgery.
Kahraman SA1. - Oral Maxillofac Surg Clin North Am. 2004 May;16(2):277-88. () 2918
View Resource
Abstract: PMID: 18088730 [PubMed]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18088730
Lack of effect of the pulsed-dye laser in the treatment of multiple eccrine hidrocystomas: a report of two cases.
Choi JE1, Ko NY, Son SW. - Dermatol Surg. 2007 Dec;33(12):1513-5. () 2928
View Resource
Abstract: PMID: 18076622 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18076622
Adverse effects of Q-switched laser treatment of tattoos.
Holzer AM1, Burgin S, Levine VJ. - Dermatol Surg. 2008 Jan;34(1):118-22. Epub 2007 Dec 5. () 2932
View Resource
Abstract: PMID: 18053032 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18053032
Photomedicine and LLT Literature Watch.
Carroll J1. - Photomed Laser Surg. 2007 Oct;25(5):463. () 2941
View Resource
Abstract: PMID: 17975963 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17975963
Views of a type I posterior glottic stenosis before and after lysis.
Davis-Malesevich M1, Merati A. - Ear Nose Throat J. 2007 Sep;86(9):536. () 2948
View Resource
Abstract: PMID: 17970140 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17970140
[Intra-auricular laser therapy of children suffering from bronchial asthma].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2007 May-Jun;(3):5-8. () 3008
View Resource
Abstract: PMID: 17647310 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17647310
[Risks of lucrative light bundles. "Blunders in laser medicine"].
[Article in German] - MMW Fortschr Med. 2006 Nov 2;148(44):16. () 3011
View Resource
Abstract: PMID: 17632868 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17632868
[Laser boom in Germany. Who guarantees quality? (interview by Dr. med. Horst Gross)].
[Article in German] - MMW Fortschr Med. 2006 Nov 2;148(44):17. () 3016
View Resource
Abstract: PMID: 17619433 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17619433
[Visit in laser surgery. Flashes for health and esthetics].
[Article in German] - MMW Fortschr Med. 2006 Nov 2;148(44):10-2, 14. () 3018
View Resource
Abstract: PMID: 17619431 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17619431
Photomedicine and LLLT LiteratureWatch.
Carroll J1. - Photomed Laser Surg. 2007 Jun;25(3):233-4. () 3020
View Resource
Abstract: PMID: 17603866 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17603866
Management of mild to moderate rhinophyma with a 1,450-nm diode laser: report of five patients.
Apikian M1, Goodman GJ, Roberts S. - Dermatol Surg. 2007 Jul;33(7):847-50. () 3034
View Resource
Abstract: PMID: 17598853 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17598853
Redarkening of port-wine stains 10 years after laser treatment.
Nelson JS, Geronemus RG. - N Engl J Med. 2007 Jun 28;356(26):2745-6; author reply 2746. () 3035
View Resource
Abstract: PMID: 17596612 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17596612
Pulsed-dye laser treatment of nonhealing chronic ulcer with hypergranulation tissue.
Wang SQ1, Goldberg LH. - Arch Dermatol. 2007 Jun;143(6):700-2. () 3038
View Resource
Abstract: PMID: 17576934 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17576934
[Magnetolaser therapy of patients with bronchial asthma in combination with essential hypertension].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2007 Mar-Apr;(2):4-7. () 3043
View Resource
Abstract: PMID: 17563978 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17563978
Fractional photothermolysis for the treatment of adult colloid milium.
Marra DE1, Pourrabbani S, Fincher EF, Moy RL. - Arch Dermatol. 2007 May;143(5):572-4. () 3056
View Resource
Abstract: PMID: 17515508 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17515508
Fractional photothermolysis for the treatment of adult colloid milium.
Marra DE1, Pourrabbani S, Fincher EF, Moy RL. - Arch Dermatol. 2007 May;143(5):572-4. () 3058
View Resource
Abstract: PMID: 17515508 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17515508
Single-cell analysis of protein kinase C activation during anti-apoptosis and apoptosis induced by laser irradiation.
Wu M1, Cheng-Yi Liu T. - Photomed Laser Surg. 2007 Apr;25(2):129-30. () 3059
View Resource
Abstract: PMID: 17508850 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17508850
[Technologies of laser prophylaxis of depressive disorder relapses].
[Article in Russian] - Voen Med Zh. 2007 Feb;328(2):31-4. () 3067
View Resource
Abstract: PMID: 17508608 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17508608
[Nonpharmalogical correction of respiratory disorders in poor health children].
[Article in Russian] - Vestn Otorinolaringol. 2006;(6):59-62. () 3091
View Resource
Abstract: PMID: 17419509 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17419509
Utilization of an Er,Cr:YSGG laser for the removal of all-ceramic restorations.
Broome PJ1. - Pract Proced Aesthet Dent. 2007 Jan-Feb;19(1):23-5. () 3094
View Resource
Abstract: PMID: 17402628 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17402628
Minimally invasive therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia.
Elhilali MM. - J Urol. 2007 Mar;177(3):820-1. () 3131
View Resource
Abstract: PMID: 17296350 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17296350
Re: "low-level laser therapy and lateral epicondylitis" Maher S. Phys Ther. 2006;86:1161-1167.
Bjordal JM. - Phys Ther. 2007 Feb;87(2):224-5; author reply 225-6. () 3141
View Resource
Abstract: PMID: 17272683 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17272683
[Current potentialities of laser radiation in otorhinolaryngological practice].
[Article in Russian] - Vestn Otorinolaringol. 2006;(5):59-62. () 3142
View Resource
Abstract: PMID: 17278698 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17278698
[Individual prognosis and identification of patient reactions on laser therapy course according to the Luscher's test].
[Article in Russian] - Voen Med Zh. 2006 Oct;327(10):32-8. () 3153
View Resource
Abstract: PMID: 17236670 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17236670
Pyogenic granuloma: satellitosis after carbon dioxide laser vaporization resolved with an intense pulsed light system.
Paradela S1, del Pozo J, MartÃnez W, Fernández-Jorge B, Rodriguez-Lozano J, Yebra-Pimentel T, Fonseca E. - Dermatol Surg. 2007 Jan;33(1):104-8. () 3157
View Resource
Abstract: PMID: 17214689 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17214689
Clearance of multiple venous lakes with an 800-nm diode laser: a novel approach.
Wall TL1, Grassi AM, Avram MM. - Dermatol Surg. 2007 Jan;33(1):100-3. () 3158
View Resource
Abstract: PMID: 17214688 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17214688
[Effects of physical factors on morphofunctional condition of cell cultures].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2006 Nov-Dec;(6):48-52. () 3160
View Resource
Abstract: PMID: 17201227 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17201227
Lasers, lights and related technologies: a review of recent journal highlights.
Ee HL1, Barlow RJ. - Clin Exp Dermatol. 2007 Jan;32(1):135-7. Epub 2006 Nov 30. () 3172
View Resource
Abstract: PMID: 17163958 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17163958
[Personalized laser therapy of cardiological patients as a pilot design of the concept of personalized physiotherapy].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2006 Sep-Oct;(5):34-8. () 3179
View Resource
Abstract: PMID: 17144562 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17144562
Physiotherapy applied to the horse: a review.
Buchner HH1, Schildboeck U. - Equine Vet J. 2006 Nov;38(6):574-80. () 3182
View Resource
Abstract: PMID: 17124850 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17124850
[Non-ablative laser refractive interventions into the fibrous tunic of the eye].
[Article in Russian] - Vestn Oftalmol. 2006 Sep-Oct;122(5):51-4. () 3200
View Resource
Abstract: PMID: 17087041 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17087041
Long-pulsed dye laser for the treatment of erythromelanosis follicularis faciei: report of two clinical cases.
Kurita M1, Momosawa A, Ozaki M, Ban I, Harii K. - Dermatol Surg. 2006 Nov;32(11):1414-7. () 3201
View Resource
Abstract: PMID: 17083598 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17083598
Lasers, light sources, and radiofrequency devices for skin rejuvenation.
Narurkar VA1. - Semin Cutan Med Surg. 2006 Sep;25(3):145-50. () 3219
View Resource
Abstract: PMID: 17055394 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17055394
Ineffective dose and lack of laser output testing in laser shoulder and neck studies.
Bjordal JM, Baxter GD. - Photomed Laser Surg. 2006 Aug;24(4):533-4; author reply 534. () 3232
View Resource
Abstract: PMID: 16995282 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16995282
Short term beneficial effects of low level laser therapy for patients with rheumatoid arthritis.
Juhl C1. - Aust J Physiother. 2006;52(3):224. () 3246
View Resource
Abstract: PMID: 16942458 [PubMed]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16942458
Low level laser therapy is not low.
Lubart R. - Photomed Laser Surg. 2006 Aug;24(4):532; author reply 532-3. () 3247
View Resource
Abstract: PMID: 16942437 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16942437
Improvement of familial benign pemphigus after treatment with pulsed-dye laser: a case report.
Fisher GH1, Geronemus RG. - Dermatol Surg. 2006 Jul;32(7):966-8. () 3274
View Resource
Abstract: PMID: 16875483 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16875483
[Prevention and treatment of face herpes with magnetic-laser radiation from Optodan unit].
[Article in Russian] - Stomatologiia (Mosk). 2006;85(3):78-82. () 3287
View Resource
Abstract: PMID: 16858330 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16858330
[Laser therapy in rehabilitation of patients with primary chronic gastroduodenitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2006 Mar-Apr;(2):10-3. () 3309
View Resource
Abstract: PMID: 16752818 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16752818
[Possibilities and prospects of the dental apparatus Optodan use for the magnetic-laser treatment of stomatologic diseases].
[Article in Russian] - Stomatologiia (Mosk). 2006;85(2):68-72. () 3318
View Resource
Abstract: PMID: 16710285 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16710285
The efficacy of low level laser therapy for chronic neck pain.
Bot SD, Bouter LM. - Pain. 2006 Sep;124(1-2):5-6. Epub 2006 May 11. () 3326
View Resource
Abstract: PMID: 16697111 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16697111
Lasers may induce terminal hair growth.
Bouzari N, Firooz AR. - Dermatol Surg. 2006 Mar;32(3):460. () 3335
View Resource
Abstract: PMID: 16640698 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16640698
Long-pulsed neodymium:yttrium-aluminum-garnet laser treatment for port wine stains.
Geronemus RG. - J Am Acad Dermatol. 2006 May;54(5):923. () 3337
View Resource
Abstract: PMID: 16635693 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16635693
Wrong parameters can give just any results.
Hode L, Tunér J. - Lasers Surg Med. 2006 Apr;38(4):343. () 3357
View Resource
Abstract: PMID: 16568446 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16568446
Laser acupuncture studies should not be included in systematic reviews of phototherapy.
Chow R. - Photomed Laser Surg. 2006 Feb;24(1):69. () 3374
View Resource
Abstract: PMID: 16503792 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16503792
[Free radical processes in the etiology, pathogenesis, and treatment of malignant tumors of the brain].
[Article in Russian] - Zh Vopr Neirokhir Im N N Burdenko. 2005 Jul-Sep;(3):39-43. () 3381
View Resource
Abstract: PMID: 16485827 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16485827
Multiple eccrine hidrocystomas: successful treatment with the 595 nm long-pulsed dye laser.
Lee HW1, Lee DK, Lee HJ, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. - Dermatol Surg. 2006 Feb;32(2):296-7. () 3398
View Resource
Abstract: PMID: 16442058 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16442058
Effect of auricular acupuncture with low power laser on four chronic allergic dermatoses and serum IgE level.
Hou YH1, Xu F, Wu SX. - Chin Med Sci J. 2005 Dec;20(4):281. () 3404
View Resource
Abstract: PMID: 16422262 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16422262
[Infrared laser therapy in combined therapy of patients with chronic salpingo-oophoritis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2005 Nov-Dec;(6):20-3. () 3409
View Resource
Abstract: PMID: 16404921 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16404921
Age-related macular degeneration: options for earlier detection and improved treatment.
Wiggins MN1, Uwaydat SH. - J Fam Pract. 2006 Jan;55(1):22-7. () 3420
View Resource
Abstract: PMID: 16388763 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16388763
Low level laser therapy is not low.
Enwemeka CS. - Photomed Laser Surg. 2005 Dec;23(6):529-30. () 3438
View Resource
Abstract: PMID: 16356141 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16356141
Acne, lasers, and light.
Ross EV1. - Adv Dermatol. 2005;21:1-32. () 3439
View Resource
Abstract: PMID: 16350436 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16350436
595 nm pulsed dye laser for the treatment of superficial basal cell carcinoma.
Campolmi P, Mavilia L, Bonan P, Cannarozzo G, Lotti TM. - Lasers Med Sci. 2005 Dec;20(3-4):147-8. Epub 2005 Nov 18. () 3443
View Resource
Abstract: PMID: 16328096 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16328096
Standards for laser therapy studies.
Tunér J, Hode L. - J Wound Care. 2005 Nov;14(10):478-9; author reply 478. () 3446
View Resource
Abstract: PMID: 16304923 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16304923
The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology.
Complications of Age-Related Macular Degeneration Prevention Trial Study Group. - Clin Trials. 2004 Feb;1(1):91-107. () 3455
View Resource
Background: The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) is a randomized clinical trial to evaluate whether prophylactic laser treatment to the retina can prevent the complications of the advanced stage of Age-Related Macular Degeneration (AMD), the leading cause of irreversible blindness.
Abstract: PMID: 16281465 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: CAPT is conducted in 23 clinical centers and in three central resource centers. The primary outcome measure is change in visual acuity; secondary outcomes include the incidence of complications of AMD, changes in other measures of visual functioning and vision-related quality of life. In total, 1052 patients with two high-risk eyes were enrolled. One eye was randomized to receive laser treatment and the other eye to observation. All patients were treated immediately after randomization and again at 12 months, dependent on clinical status. All patients are followed via study visits and telephone calls for a minimum of five years. Study visit procedures include established tests of visual function conducted by examiners masked to the treatment assignment of each eye, a biomicroscopic examination by CAPTophthalmologists, and photographs of each eye taken according to protocol and assessed by masked graders in a centralized Photograph Reading Center.
Results: This paper describes the CAPT study, including study rationale, operational structure, and measures implemented to ensure standardization of assessments, adherence to protocol, quality assurance, and maintaining follow-up. Several features related to study design and procedures that are specific to CAPT are highlighted, including clinic selection and judgements regarding patient eligibility.
Conclusions: An intervention that can reduce the risk of advanced AMD by 30% in the eyes of people with two high-risk eyes may halve the rate of bilateral blindness from AMD. It would also yield substantial savings in expenditures devoted to treating advanced AMD and the disability it causes, and enhance the quality of life for people at risk.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16281465
Pure or tarnished: are systematic reviews blind or biased?
Lanzafame RJ. - Photomed Laser Surg. 2005 Oct;23(5):451-2. () 3464
View Resource
Abstract: PMID: 16262572 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16262572
[Quality and documentation prior to dermatological laser treatment].
[Article in Danish] - Ugeskr Laeger. 2005 Oct 24;167(43):4063. () 3470
View Resource
Abstract: PMID: 16251087 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16251087
C225 and PDT combination therapy for ovarian cancer: the play's the thing.
Cengel KA, Hahn SM, Glatstein E. - J Natl Cancer Inst. 2005 Oct 19;97(20):1488-9. () 3476
View Resource
Abstract: PMID: 16234556 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16234556
Physical and rehabilitative approaches in osteoarthritis.
Di Domenica F1, Sarzi-Puttini P, Cazzola M, Atzeni F, Cappadonia C, Caserta A, Galletti R, Volontè L, Mele G. - Semin Arthritis Rheum. 2005 Jun;34(6 Suppl 2):62-9. () 3484
View Resource
Abstract: PMID: 16206961 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16206961
The importance of the coherency.
Hode L. - Photomed Laser Surg. 2005 Aug;23(4):431-4. () 3513
View Resource
Abstract: PMID: 16144489 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16144489
Effects of an infrared pulsed laser device (IPLD) over apoptosis in cancer cells.
Santana-Blank L. - Bioelectromagnetics. 2005 Sep;26(6):523-4. () 3532
View Resource
Abstract: PMID: 16108041 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16108041
[Combined use of low-intensive laser therapy and biologically active food additive Fitopan M in sanatorium treatment of patients with chronic prostatitis].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2005 May-Jun;(3):35-6. () 3537
View Resource
Abstract: PMID: 16060283 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16060283
Successful treatment of recalcitrant pemphigus vulgaris and pemphigus vegetans with etanercept and carbon dioxide laser.
Lin MH1, Hsu CK, Lee JY. - Arch Dermatol. 2005 Jun;141(6):680-2. () 3558
View Resource
Abstract: PMID: 15967912 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15967912
[Reflexotherapy used in excretory-and-inflammatory form of male infertility].
[Article in Russian] - Voen Med Zh. 2005 Apr;326(4):35-8. () 3565
View Resource
Abstract: PMID: 15962599 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15962599
Low-level laser therapy.
Kleinkort J1. - Rehab Manag. 2005 Jun;18(5):51. () 3567
View Resource
Abstract: PMID: 15957498 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15957498
[Low intensive infrared laser radiation in early rehabilitation of patients after acute disorders of cerebral circulation (guidelines for physicians)].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2005 Mar-Apr;(2):49-53. () 3579
View Resource
Abstract: PMID: 15916378 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15916378
[Changes in erythrocyte count during blood laser radiation].
[Article in Russian] - Voen Med Zh. 2005 Mar;326(3):28-31. () 3597
View Resource
Abstract: PMID: 15864963 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15864963
[The efficiency of magneto-laser therapy used in excretory-and-inflammatory form of male infertility].
[Article in Russian] - Voen Med Zh. 2005 Feb;326(2):38-41. () 3607
View Resource
Abstract: PMID: 15822776 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15822776
[Laser therapy in the combined treatment of pulmonary tuberculosis in adolescents (a guide for the physician)].
[Article in Russian] - Probl Tuberk Bolezn Legk. 2005;(1):56-61. () 3610
View Resource
Abstract: PMID: 15801642 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15801642
[Preventing locomotory diseases caused by static, dynamic load on hands and by local vibration].
[Article in Russian] - Med Tr Prom Ekol. 2004;(12):41-3. () 3623
View Resource
Abstract: PMID: 15773387 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15773387
[New treatment methods in pathologies of the choroid and retina including the use of subthreshold power of diode infrared laser radiation].
[Article in Russian] - Vestn Oftalmol. 2005 Jan-Feb;121(1):49-54. () 3630
View Resource
Abstract: PMID: 15759854 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15759854
Principles of cosmetic dentistry in orthodontics: Part 3. Laser treatments for tooth eruption and soft tissue problems.
Sarver DM1, Yanosky M. - Am J Orthod Dentofacial Orthop. 2005 Feb;127(2):262-4. () 3633
View Resource
Abstract: PMID: 15750548 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15750548
[Laser treatment supposedly modifies dentin. 1 visit to the dentist--an no caries from then on? (interview by Dr. Thomas Meissner)].
[Article in German] - MMW Fortschr Med. 2005 Jan 20;147(3):16. () 3634
View Resource
Abstract: PMID: 15727104 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15727104
Lupus miliaris disseminatus faciei: treatment with the 1450-nm diode laser.
Jih MH1, Friedman PM, Kimyai-Asadi A, Friedman ES, Hymes SR, Goldberg LH. - Arch Dermatol. 2005 Feb;141(2):143-5. () 3637
View Resource
Abstract: PMID: 15724009 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15724009
[Male infertility treatment using KAP-ELM-01 apparatus complex "Andro-Gin"].
[Article in Russian] - Urologiia. 2004 Nov-Dec;(6):34-6. () 3639
View Resource
Abstract: PMID: 15719728 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15719728
Lasers in dermatology: two-year experience at University Department of Dermatology and Venerology, Zagreb University Hospital Center, Zagreb, Croatia.
Buković-Mokos Z1. - Acta Dermatovenerol Croat. 2004;12(4):314. () 3644
View Resource
Abstract: PMID: 15714675 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15714675
[Laser technologies used in the complex treatment of psychopharmacotherapy resistant endogenic depression].
[Article in Russian] - Voen Med Zh. 2004 Nov;325(11):37-42. () 3664
View Resource
Abstract: PMID: 15675751 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15675751
Rosacea in a new light.
Romagnolo SC1, Benedetto AV. - Skinmed. 2005 Jan-Feb;4(1):47-8. () 3674
View Resource
Abstract: PMID: 15654166 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15654166
Resolution of retracted scar after 585-nm pulse dye laser surgery.
Lack EB, Rachel JD. - J Cosmet Laser Ther. 2004 Nov;6(3):149-51. () 3696
View Resource
Background: Laser scar revision has been an effective method for improving several aspects of scarring through ablative and non-ablative sources. The 585-nm pulsed dye laser (PDL) is an important non-ablative instrument for reducing scar bulk and symptoms.
Abstract: PMID: 15545099 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Methods: To describe the use of a 585-nm PDL for the treatment of a retracted and atrophic facial scar.
Results: We report the case of a 26-year-old patient who presented with a retracted facial scar following surgical excision of an aggressive benign tumor. Treatment was carried out using the 585-nm PDL.
Conclusions: Treatment of the scar using two low-level PDL therapies significantly altered the appearance of the scar and augmentation of the retracted defect was avoided.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15545099
Ottawa Panel Evidence-Based Clinical Practice Guidelines for Electrotherapy and Thermotherapy Interventions in the Management of Rheumatoid Arthritis in Adults.
Ottawa Panel. - Phys Ther. 2004 Nov;84(11):1016-43. () 3702
View Resource
Background: The purpose of this project was to create guidelines for electrotherapy and thermotherapy interventions in the management of adult patients (>18 years of age) with a diagnosis of rheumatoid arthritis according to the criteria of the American Rheumatism Association (1987).
Abstract: PMID: 15509188 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Methods: Using Cochrane Collaboration methods, the Ottawa Methods Group identified and synthesized evidence from comparative controlled trials. The group then formed an expert panel, which developed a set of criteria for grading the strength of the evidence and the recommendation. Patient-important outcomes were determined through consensus, provided that these outcomes were assessed with a validated and reliable scale.
Results: The Ottawa Panel developed 8 positive recommendations of clinical benefit. Lack of evidence meant that the panel could not gauge the efficacy of electrical stimulation.
Conclusions: The Ottawa Panel recommends the use of low-level laser therapy, therapeutic ultrasound, thermotherapy, electrical stimulation, and transcutaneous electrical nerve stimulation for the management of rheumatoid arthritis.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15509188
[Efficacy of low-intensive laser irradiation in coronary heart disease].
[Article in Russian] - Ter Arkh. 2004;76(8):79-82. () 3710
View Resource
Abstract: PMID: 15471404 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15471404
[Quality of life as a research field in physiotherapy].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2004 Jul-Aug;(4):38-43. () 3715
View Resource
Abstract: PMID: 15449674 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15449674
Pulsed dye laser treatment of acne vulgaris.
Chu AC. - JAMA. 2004 Sep 22;292(12):1430; author reply 1430. () 3721
View Resource
Abstract: PMID: 15383510 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15383510
[Effect of laser therapy on the patient respiration system in the acute phase of inhalation burn].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2004 May-Jun;(3):18-20. () 3769
View Resource
Abstract: PMID: 15216785 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15216785
308-nm excimer laser for the treatment of scalp psoriasis.
Gupta SN1, Taylor CR. - Arch Dermatol. 2004 May;140(5):518-20. () 3797
View Resource
Abstract: PMID: 15148093 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15148093
[Characteristics of the rehabilitation in patients with facial trauma of middle and upper regions and lesions in the locomotor and muscular apparatus of the eye and orbit].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2004 Jan-Feb;(1):25-8. () 3822
View Resource
Abstract: PMID: 15052842 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15052842
[Acute catarrhal appendicitis: is appendectomy required?].
[Article in Russian] - Khirurgiia (Mosk). 2004;(2):69-72. () 3840
View Resource
Abstract: PMID: 14997890 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14997890
[Evaluation of low level laser therapy in temporo-mandibular joint disorders].
[Article in Chinese] - Shanghai Kou Qiang Yi Xue. 2003 Dec;12(6):435, 442. () 3841
View Resource
Abstract: PMID: 14966584 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14966584
Therapeutic light.
Enwemeka CS1. - Rehab Manag. 2004 Jan-Feb;17(1):20-5, 56-7. () 3842
View Resource
Abstract: PMID: 14974136 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14974136
[Laser infrared irradiation in the complex treatment of gastroduodenal ulcer].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 Nov-Dec;(6):33-5. () 3855
View Resource
Abstract: PMID: 14753013 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14753013
[Possibilities of the low-intensity infrared laser therapy in atherosclerotic lesions of the cardiovascular system].
[Article in Russian] - Vestn Ross Akad Med Nauk. 2003;(12):45-52. () 3859
View Resource
Abstract: PMID: 14724973 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14724973
Practice of acupuncture and laser hair removal.
Weber RD1. - Mich Med. 2003 Nov-Dec;102(6):7. () 3865
View Resource
Abstract: PMID: 14677336 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14677336
CO2 laser therapy for Milia en plaque.
Sandhu K1, Gupta S, Handa S. - J Dermatolog Treat. 2003 Dec;14(4):253-5. () 3874
View Resource
Abstract: PMID: 14660275 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14660275
[Laser therapy in gastroenterology].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 Sep-Oct;(5):52-4. () 3882
View Resource
Abstract: PMID: 14650142 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14650142
[Hemangioma in the infant. Freezing, laser treatment--or waiting?].
[Article in German] - MMW Fortschr Med. 2003 Sep 11;145(37):20-2. () 3891
View Resource
Abstract: PMID: 14584438 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14584438
[Role of genetic factors in responses to low-level magnetic and laser therapy in patients with cardiac diseases].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 Jul-Aug;(4):13-20. () 3914
View Resource
Abstract: PMID: 12945136 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12945136
Pulsed dye laser treatment of superficial basal cell carcinoma: realistic or not?
Allison KP, Kiernan MN, Waters RA, Clement RM. - Lasers Med Sci. 2003;18(2):125-6. () 3918
View Resource
Abstract: PMID: 12928824 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12928824
[Chest neuropathy and thoracalgia. Physical methods of treatment].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 May-Jun;(3):50-2. () 3933
View Resource
Abstract: PMID: 12852022 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12852022
[Infrared laser therapy in complex treatment of patients with ischemic heart disease after aortocoronary bypass surgery].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 May-Jun;(3):18-21. () 3936
View Resource
Abstract: PMID: 12852009 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12852009
Office-based pulsed dye laser treatment for hemorrhagic telangiectasias and epistaxis.
Hartnick CJ1, Dailey S, Franco R, Zeitels SM. - Laryngoscope. 2003 Jun;113(6):1085-7. () 3949
View Resource
Abstract: PMID: 12782829 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12782829
[Principles of treatment and prophylaxis of chronic prostatitis].
[Article in Russian] - Voen Med Zh. 2003 Feb;324(2):27-31. () 3962
View Resource
Abstract: PMID: 12722360 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12722360
[Physiological and therapeutic value of the so-called weak impacts in physiotherapy].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2003 Jan-Feb;(1):21-5. () 3963
View Resource
Abstract: PMID: 12698702 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12698702
[Preventive use of ozone, short waves, and laser therapy alone and in combination in early postoperative period after dental implantation].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2002 Nov-Dec;(6):17-9. () 3997
View Resource
Abstract: PMID: 12592899 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12592899
[Effects of electric stimulations or low-frequency laser irradiation on the pH of the nerve].
[Article in Russian] - Fiziol Cheloveka. 2002 Nov-Dec;28(6):127-8. () 4001
View Resource
Abstract: PMID: 12564236 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12564236
Early pulsed-dye laser treatment of childhood haemangiomas.
Kolde G. - Lancet. 2003 Jan 25;361(9354):348-9; author reply 349. () 4005
View Resource
Abstract: PMID: 12559891 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12559891
Early pulsed-dye laser treatment of childhood haemangiomas.
Maier H, Donath P, Neumann R. - Lancet. 2003 Jan 25;361(9354):348; author reply 349. () 4007
View Resource
Abstract: PMID: 12559890 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12559890
[Application of the low-intensive laser irradiation for the prophylaxis of the small intestinal anastomosis sutures insufficiency].
[Article in Russian] - Klin Khir. 2002 Nov-Dec;(11-12):42. () 4010
View Resource
Abstract: PMID: 12549280 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12549280
Splinting vs surgery for carpal tunnel syndrome.
Weintraub MI. - JAMA. 2003 Jan 22-29;289(4):422; author reply 422-3. () 4014
View Resource
Abstract: PMID: 12533109 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12533109
Dermatologic history of the ruby laser: the long story of short pulses.
Anderson RR1. - Arch Dermatol. 2003 Jan;139(1):70-4. () 4016
View Resource
Abstract: PMID: 12533169 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12533169
[Effect of low intensity infrared laser radiation on cell membrane lipids in experiment].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2002 Sep-Oct;(5):28-32. () 4017
View Resource
Abstract: PMID: 12532595 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12532595
Lower-extremity varicosities: endoluminal therapy.
Min RJ1, Khilnani NM. - Semin Roentgenol. 2002 Oct;37(4):354-60. () 4042
View Resource
Abstract: PMID: 12455132 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12455132
[Low-energy laser radiation in otorhinolaryngology: history and current opportunities].
[Article in Russian] - Vestn Otorinolaringol. 2002;(4):51-4. () 4056
View Resource
Abstract: PMID: 12400143 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12400143
[Changes in oxygen affinity for hemoglobin during exposure to low frequency magnetic field and low energy laser radiation].
[Article in Russian] - Fiziol Cheloveka. 2002 Sep-Oct;28(5):134-6. () 4057
View Resource
Abstract: PMID: 12397943 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12397943
[Low-intensity laser irradiation in the treatment of patients with tuberculosis of the urinary system].
[Article in Russian] - Probl Tuberk. 2002;(6):39-41. () 4074
View Resource
Abstract: PMID: 12227049 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12227049
Migraine, tension-type headache and facial pain. A common intraoral etiology and treatment.
Friedman MH. - N Y State Dent J. 2002 Jun-Jul;68(6):24-6. () 4090
View Resource
Background: A maxillary alveolar mucosal inflammation, demonstrated by local tenderness and increased temperature, is present in migraine, tension-type headache and facial pain patients even when the patient is asymptomatic. Research is presented showing effective treatment of these conditions with fewer side effects than with standard medication by local anti-inflammatory methods. These alternative methods include: chilling, application of anti-inflammatory gel and low-level (non-cutting) laser. Local treatment also mediates cervical muscle spasm, adding to its overall effectiveness.
Abstract: PMID: 12149787 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12149787
[Recovery physiotherapy of myopathy in flying personnel of the Air Forces].
[Article in Russian] - Voen Med Zh. 2002 Apr;323(4):54-7. () 4111
View Resource
Abstract: PMID: 12046535 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12046535
Laser hair removal.
Ort RJ, Dierickx C. - Semin Cutan Med Surg. 2002 Jun;21(2):129-44. () 4114
View Resource
Background: The presence of unwanted hair continues to plague many individuals for whom traditional methods of hair removal remain unsatisfactory. Laser and flashlamp technology now offers the potential for rapid, safe, and effective treatment of unwanted hair. An ever-increasing number of published studies have confirmed the long-term efficacy of laser and flashlamp treatment. For the most part, however, the benefits of this technology have been limited to individuals with dark hair and relatively fair skin. The remaining challenge is to develop the means to eliminate light-colored hair as well as the capability to safely treat individuals with darker skin. The rapid pace of technological advancement as well as continued studies of hair follicle biology promise to improve this field over the years to come.
Abstract: PMID: 12056574 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12056574
Effect of pulsed laser radiation on regeneration of injured muscles with different regeneration capacities and the state of the thymus.
Bulyakova NV1, Zubkova SM, Azarova VS, Mikhailik LV, Varakina NI. - Dokl Biol Sci. 2002 Jan-Feb;382:65-70. () 4119
View Resource
Abstract: PMID: 11998760 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11998760
Phototherapy of psoriasis: update with practical pearls.
Lui H1. - J Cutan Med Surg. 2002 May-Jun;6(3 Suppl):17-21. Epub 2002 Apr 30. () 4127
View Resource
Abstract: PMID: 11976982 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11976982
EBM in action: is laser treatment effective and safe for musculoskeletal pain?
Chow R. - Med J Aust. 2002 Feb 18;176(4):194-5. () 4135
View Resource
Abstract: PMID: 11913929 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11913929
The irreplaceable image: Amelioration of oral mucosal lesions of acute graft-versus-host disease by low-level laser therapy.
Chor A1, Sotero Caio AB, de Azevedo AM. - Haematologica. 2001 Dec;86(12):1321. () 4184
View Resource
Abstract: PMID: 11726330 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11726330
Nonablative laser and light rejuvenation: the newest approach to photodamaged skin.
Kelly KM1, Majaron B, Nelson JS. - Arch Facial Plast Surg. 2001 Oct-Dec;3(4):230-5. () 4185
View Resource
Abstract: PMID: 11710855 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11710855
[New generation laser technology and author's patented methods for treatment of oral and dental diseases].
[Article in Russian] - Stomatologiia (Mosk). 2001;80(5):57-9. () 4188
View Resource
Abstract: PMID: 11696955 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11696955
[Laser correction of dysregulations of involutionary genesis in females and males aged 40-60 years].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2001 Jul-Aug;(4):47-8. () 4198
View Resource
Abstract: PMID: 11561309 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11561309
[Incoherent infrared therapy in the treatment of jaw inflammation diseases].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2001 May-Jun;(3):39-40. () 4202
View Resource
Abstract: PMID: 11550382 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11550382
Low level laser therapy wound treatment update.
Sliney D1. - J Laser Appl. 1999 Oct;11(5):221-4. () 4227
View Resource
Abstract: PMID: 10623342 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/10623342
Comments on the use of low-level laser therapy (LLLT) in painful musculo-skeletal disorders.
Jacobsen FM, Couppé C, Hilden J. - Pain. 1997 Oct;73(1):110-1. () 4246
View Resource
Abstract: PMID: 9414066 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/9414066
Low level laser therapy.
Gage JP. - Aust Dent J. 1997 Dec;42(6):414. () 4247
View Resource
Abstract: PMID: 9470287 [PubMed - indexed for MEDLINE] Free full text
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/9470287
An experimental study of the effect of low level helium-neon laser irradiation on the reparative processes of the cornea after perforative injury.
Zapryanov Z1, Koev K, Tanev V, Russeva M. - Folia Med (Plovdiv). 1990;32(2):39-44. () 4313
View Resource
Abstract: PMID: 2103917 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/2103917
Laser-resistant guide probe for laser treatment of endoscopically impassable tumour stenoses.
Ell C, Hochberger J, Lux G, Riemann JF. - Endoscopy. 1986 Jan;18(1):27-8. () 4318
View Resource
Background: A laser-resistant guide probe that can be placed endoscopically, for use in the laser treatment of impassable tumour stenosis is described. It meets the necessary requirements such as low level of absorption of YAG laser light (1,064 nm wavelength), high thermal stability and low heat conduction. So far, the probe has been used complication-free, in four patients.
Abstract: PMID: 3948803 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/3948803
Lyme Laser Centers of New England
Dr. Douglas A. Wine - (Publication) 4367
We are not recommending this resource but they claim guaranteed results in curing lyme disease at a reasonable price and that is worth checking out.
View Resource
At the Lyme Laser center of New England, we have successfully cared for hundreds of Acute and Chronic Lyme patients. Our research has proven that all Lyme patients suffer from undiagnosed environmental toxicity that severely suppresses their immune function. Because of their compromised immune function, Lyme patients always have underlying causes that go undiagnosed and overlooked. It is just as important to be able to find out what these issues are and address them as well as the Lyme itself. This, along with our Lyme Specific Lasers and proprietary Supplements, is one of the reasons why our success rate is better than 90 percent.
OUR TREATMENT PROTOCOLS FOR LYME DISEASE
The protocol that we have developed has been used for years on hundreds of Lyme patients with great success. Every protocol is specific to each Lyme patient. In fact, our success rate is better than 90 percent.
The #1 reason for our success is the use of cold laser therapy also known as low level lasers. Reason #2 is that our in-depth questionnaire finds the secondary and tertiary causes which allows us to address them as well as the Lyme.
“This condition is better termed Lyme MSIDS, short for Multiple Systemic Infectious Disease Syndrome. MSIDS is like Pandora’s Box because it includes many infections, co-infections and secondary infections. Treatment should be tailored to each patient individually.” Richard Horowitz, MD and author of the book, Why Can’t I Get Better? Solving the Mystery of Lyme and Chronic Disease.
We only use safe, natural, non-invasive modalities, techniques and supplements including, but not limited to:
Whole-body Laser
Specific Lyme frequency lasers
Ionic Detox
Oscillation
Anatomotor
Specific nutrition and proprietary supplements
Evaluation of all external factors including chemicals and ionizing radiation
Addressing stress issues
Addressing parasitic, bacterial, viral infections and fungus
Addressing other lifestyle factors including EMF, microwaves, wireless technologies, etc.
SPECIFIC LOW LEVEL/COLD LASERS
The wave of the future is these new lasers. Dr. Wine has developed protocols and different types of these specific lasers since 1995. These non-invasive lasers have the ability to significantly accelerate and enhance the body’s natural defense and repair components to increase your health potential. Cold lasers deliver the required energy directly to the cells which enhance their ability to produce ATP (adenosine-tri-phosphate) – which is necessary for optimal function, cell repair and regeneration, healing, weight loss and endorphin production, which are the body’s natural pain killers.
Laser therapy has a direct effect on immunity status by stimulation of immunoglobins and lymphocytes. Laser light is absorbed by chromophones (molecule enzymes) that react to laser light. The enzyme flavomono-nucleotide is activated and starts the production of ATP, which is the major carrier of cell energy and the energy source for all chemical reactions in the cells.
Original Source: http://www.lymelasercentersofnewengland.com/
Interview with Wie Chen
Wie Chen - Phone interview 5/3/18 (Web) 4477
Dr. Wie Chen says to never treat an area with cancer with a non-thermal dosage of LLLT.
View Resource
ColdLasers recently sold a system to Dr. Wie Chen. He has numerous publications, including several with Dr Hamblin and others and is a leading researcher in the use of higher power lasers for cancer treatment. During the interview, he reviewed how he uses high powered laser to thermally destroy cancer followed up by a special program to promote normal healing. This is done by increasing the tissue temperature to around 60 degree C (140 degree F). This is not using the laser to cut out the cancer but to thermally destroy the damaged cells.
During the interview, I ask if non-thermal dosages of LLLT can help cancer grow. He said that they do see growth in the cancer in tissue sample with non-thermal (low intensity) LLLT.
We are adding this “non-published” information to the library as a contrary position to the studies that show there is no interaction with cancer and LLLT. We have heard from some manufacturer, that LLLT only grows healthy cells but Dr Chen’s research conflicts with this information.
[Study of microcirculation with laser Doppler flowmetry in patients with facial nerve neuritis under the influence of physiotherapy].
[Article in Russian] - Vopr Kurortol Fizioter Lech Fiz Kult. 2004 Mar-Apr;(2):40-1. (Publication) 3796
View Resource
Abstract: PMID: 15154357 [PubMed - indexed for MEDLINE]
Share on Facebook
Share on Twitter
Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15154357
Home Search Introduction
Ken Teegardin - (Website) 4361
View Resource
This tool is a searchable collection of technical publications, books, videos and other resources about the use of lasers and light for PhotoBioModulation (PBM). Enter a keyword above or see some of our favorite queries below.
Here are some of our favorite queries:
- Information for Beginners
- Best wavelength for PBM (980nm vs. 810nm vs 660nm...)
- Compare Lasers and LEDs
- Pulsing versus Continuous Wave (CW)
- Dosage: The best practices for dosage
- How NOT to promote laser therapy (by Turner and Hode)
- Great summary of positive double blind studies
- Whole Body Systems
- See all videos
- See all books about laser therapy and cold lasers
- Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing Athletic Performance
- Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Intro to laser acupuncture
- Traumatic brain injury research
- Soft Tissue
- Autoimmune
- Lyme's Disease
- Parkinson's research
- Alzheimer's research
- Hashimoto's research
- Covid
- Contraindications
- LLLT and Cancer
All the resources include links to the original source so we are not making any statement about the use of lasers for treating non-FDA cleared application, we are simple summarizing what others have said.
Where every possible, we have included a link to the orginal publication.
This tool uses a broad match query so:
- It does not correct spelling and searches only cold laser related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- Where ever possible, the detailed section about the resource will link to the sources.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic lasers. It does include some resources on weight loss and smoking cessation.
The results of the search are sorted based on 3 quality factors on a scale of 1 to 10 with 10 being the best score. Originally all the resources were given a 5-5-5 until they could be individually evaluated. These scores are purely opinion and are only used to simplify the rank of the results from more valuable to least valuable. This should not be considered a critique of any work. This system was created to help researchers (including ourselves) find the most usable resources for any cold laser therapy research. The resources are assigned values based on the following 3 factors:
- Efficacy: The resource (especially research papers) should show a significant improvement in the condition being treated. Resources that show better results are given a higher quality score.
- Detail: The source must give enough information that the results can be duplicated. If a resource lacks too many details that it cannot be recreated, it is given a lower detail score.
- Lack of Bias: Many resources are created to try and show that one device is superior to its competition. Many manufacturers have staff that crank out biased papers on a regular basis on the hope that this will make their product look superior. If the author of the resource is paid by a manufacturer of the resource appears to be biased towards one device and not one technology, the resource has much less value.
Over the past few years of working with research, we found that a majority of the published resources are lacking in one of these three ranking factors.
The original goal of this research tool was to tie published resources to the protocols in the laser-therapy.us library. This connection allows users to trace each protocol back to a list of resources so the protocol can be researched and improved.
General Comments
POWER
When many of the first research papers were published, the most power laser available for therapy were less than 100mW and many systems had to be pulsed to keep the laser from burning out too quickly. Today, system are available that will deliver up to 60,000mW of continuous output. Because of these power limitation, many early studies were limited to extremely low dosages by today’s standards. It takes a 50mW system 17 minutes to deliver 50 joules at the surface of the skin. If this was spread over a large area of damage or was treating a deeper problem, the actual dosages were much less than 1J/cm2. Today, we know that these dosages typically produce very little or no results.
WAVELENGTH
About 80% of the resources in this database are in the near infrared wavelength. There is also some interest in the red wavelength (600 to 660nm) . Other wavelengths like blue, purple, and green have very little scientific research behind them and have not gotten much traction in the core therapy market with the exception of some fringe consumer products.
Legal Disclaimer
This research tool is free to use but we make no claims about the accuracy of the information. It is an aggregation of existing published resources and it is up to the user to determine if the source of the resources has any value. The information provided through this web site should not be used for diagnosing or treating a health problem or disease. If you have or suspect you may have a health problem, you should consult your local health care provider.
Biophtonica Introduction
Biophotonica - (Website) 4523
View Resource
Welcome to the BioPhotonica Education Center. There are over 5000 successful studies showing the efficacy of PBM, light therapy and sound therapy. This is a searchable collection of technical publications, books, videos and other resources about the best practices in the industry and about treating a wide variety of problems. All the resources include links to the original source (where available) so we are not making any claims about the use of our technology for treating "non-FDA cleared" applications, we are simply summarizing what the expert are saying about proper application of these technologies.
Enter a keyword above and click on one of the following links to see a set of publications about that subject. HINT: Shorter keywords work better.
Here are some of our favorite queries:
- Whole Body Systems
- Treating Post Covid with PBMT
- Info for beginners (See several graphic representation of the PBM chemical process)
- Best wavelength for light therapy (980nm vs. 810nm vs 660nm...)
- Pulsing Versus continuous wave
- Dosage: Searching for the best practices for dosage
- Great summary of positive double blind studies
Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing athletic performance
Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Laser acupuncture
- Traumatic Brain Injury (TBI)
- Soft Tissue Injury
- Fertility and Reproductive Health
Autoimmune Research
Contraindications
This tool uses a broad match query so:
- It does not correct spelling and searches only PBM related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic therapies.
Light House Health Introduction
LightHouse - (Website) 4515
View Resource
Welcome to the Lighthouse Health Education Center. There are over 5000 successful studies showing the efficacy of PBM, light therapy and sound therapy. This is a searchable collection of technical publications, books, videos and other resources about the best practices in the industry and about treating a wide variety of problems. All the resources include links to the original source (where available) so we are not making any claims about the use of our technology for treating "non-FDA cleared" applications, we are simply summarizing what the expert are saying about proper application of these technologies.
Enter a keyword above and click on one of the following links to see a set of publications about that subject. HINT: Shorter keywords work better.
Here are some of our favorite queries:
- Whole Body Systems
- Treating Post Covid with PBMT
- Info for beginners (See several graphic representation of the PBM chemical process)
- Best wavelength for light therapy (980nm vs. 810nm vs 660nm...)
- Pulsing Versus continuous wave
- Dosage: Searching for the best practices for dosage
- Great summary of positive double blind studies
Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing athletic performance
Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Laser acupuncture
- Traumatic Brain Injury (TBI)
- Soft Tissue Injury
Autoimmune Research
Contraindications
This tool uses a broad match query so:
- It does not correct spelling and searches only cold laser related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic therapies.
The query result(s) can be shared using the following direct link. Anyone who clicks on this link in an email or on a web site will be shown the current results for the query.
https://www.laser-therapy.us/research/index.cfm?researchinput=book
Treatments for Traumatic Brain Injury With Emphasis on Transcranial Near-Infrared Laser Phototherapy
Larry D Morries, Paolo Cassano, Theodore A Henderson, - This article was published in Neuropsychiatric Disease and Treatment, 20 August 2015 (Publication) 4406This exceptional research indicated prefered wavelenghts and dosages for treating patients with traumatic brain injuries. The found some surprising results.
Abstract:
Traumatic brain injury (TBI) is a growing health concern affecting civilians and military personnel. In this review, treatments for the chronic TBI patient are discussed, including pharmaceuticals, nutraceuticals, cognitive therapy, and hyperbaric oxygen therapy. All available literature suggests a marginal benefit with prolonged treatment courses. An emerging modality of treatment is near-infrared (NIR) light, which has benefit in animal models of stroke, spinal cord injury, optic nerve injury, and TBI, and in human trials for stroke and TBI. The extant literature is confounded by variable degrees of efficacy and a bewildering array of treatment parameters. Some data indicate that diodes emitting low-level NIR energy often have failed to demonstrate therapeutic efficacy, perhaps due to failing to deliver sufficient radiant energy to the necessary depth. As part of this review, we present a retrospective case series using high-power NIR laser phototherapy with a Class IV laser to treat TBI. We demonstrate greater clinical efficacy with higher fluence, in contrast to the bimodal model of efficacy previously proposed. In ten patients with chronic TBI (average time since injury 9.3 years) given ten treatments over the course of 2 months using a high-power NIR laser (13.2 W/0.89 cm2 at 810 nm or 9 W/0.89 cm2 at 810 nm and 980 nm), symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability improved. Symptoms were monitored by depression scales and a novel patient diary system specifically designed for this study. NIR light in the power range of 10-15 W at 810 nm and 980 nm can safely and effectively treat chronic symptoms of TBI. The clinical benefit and effects of infrared phototherapy on mitochondrial function and secondary molecular events are discussed in the context of adequate radiant energy penetration. Keywords: infrared, traumatic brain injury, TBI, transcranial infrared light therapy, transcranial laser therapy
INTRODUCTION
Traumatic brain injury (TBI) has recently moved into the limelight due to the recognition of its impact on professional athletes and military personnel. Yet, TBI is neither a new problem nor limited to those two populations. The Centers for Disease Control and Prevention estimated that 1.5 million Americans sustained TBI annually in 2000.1 As of 2006, the estimates had risen to 1.7 million brain injuries annually.2,3 Undoubtedly, these point prevalence proportions will increase as military personnel return home,4 and the problem of repeated mild TBI (mTBI) becomes more recognized in sports.5 Current estimates of the prevalence of TBI among veterans range from 9.6%6 to 20%,7 with an estimated total of more than 300,000 cases of TBI among military personnel since 2000.4 The current estimates of the combined number of sportsrelated concussions and brain injuries in the US are 1.6-3.8 million annually.8-10 TBI results in a wide spectrum of neurological, psychiatric, cognitive, and emotional consequences. In part, the variation is related to the severity of the injury (mild, moderate, severe TBI), which is stratified based on Glasgow Coma score, periods of unconsciousness, and degrees of amnesia. Furthermore, the diversity of sequalae can be related to the areas of the brain that are injured, the severity of the injury (highly variable within the classification of “mild” and “moderate”), and the evolution of the injury over time due to neuroinflammatory processes.11,12 Additional mechanisms thought to underlie the damage of TBI include decreased mitochondrial function, calcium and magnesium dysregulation, excitotoxicity, disruption of neural networks, free radicalinduced damage, excessive nitric oxide, ischemia, and damage to the blood-brain barrier. Together, these can contribute to a progression of the damage over time. Patients with TBI can experience headache, visual disturbances, dizziness, cognitive impairment, loss of executive skills, memory impairment, fatigue, impulsivity, impaired judgment, emotional outbursts, anxiety, and depression.3,13-23 The situation can be further clouded by secondary and/ or comorbid posttraumatic stress disorder (PTSD), depression, and anxiety,17-25 which can have symptoms that overlap with those described above and appear to be increasingly likely with repetitive concussive or subconcussive brain injury.5,24,26
TREATMENTS FOR TBI
Pharmacological treatments Pharmacological treatment largely targets the neuropsychiatric sequalae of TBI, rather than providing any means of healing or repairing injury. In general, pharmacological treatment is focused on the modulation of major neurotransmitter systems – dopaminergic, serotonergic, noradrenergic, acetylcholinergic, and glutaminergic.20 Disruption of the major neurotransmitter pathways may result from direct injury or excitotoxicity and other cytotoxic mechanisms. The treatment of depression secondary to TBI is often approached with serotonin reuptake inhibitors. Several studies have examined the benefit of sertraline in post- TBI depression.27-29 Other serotonin reuptake inhibitors also have been examined. Tricyclic antidepressants appear to have some use in the treatment of post-TBI depression, although cautious dose titration is required. Patients with TBI are at greater vulnerability to sedation and cholinergic side effects of confusion and memory impairment. With serotonergic agents other than sertraline, cognitive effects also have been reported.30 Similarly, lithium may be a less desirable agent in this population due to sedation and cognitive impairment. Patients with TBI may respond at lower doses and lower blood levels than expected. Modulation of the dopaminergic system may improve alertness, attention, and cognitive processing speed. The stimulants are most commonly used for this purpose. Methylphenidate facilitates the release of dopamine and slows its reuptake. Dextroamphetamine strongly inhibits reuptake of dopamine, slows down the breakdown of dopamine by monoamine oxidase, and somewhat increases the release of dopamine. These subtle differences are sometimes imperceptible to the patient, but at other times, a patient will do best on one or the other stimulant. Increasing dopamine in the reticular activating system leads to enhanced arousal. Increasing dopamine within the frontal cortex and the striatum leads to enhanced processing speed and attention. Some evidence suggests that the stimulants may enhance neuronal recovery after injury.31-33 There are numerous potential side effects with stimulants, including abnormal heart rhythms, decreased seizure threshold, and death, but these severe side effects are extremely rare. The most common side effects with stimulants are decreased appetite, stomach upset, and headache. These are most severe at the beginning of treatment and improve over time for most patients. Insomnia is another common side effect, which may be more frequent in those with a TBI. Amantadine and bromocriptine may also increase dopamine. Studies of these agents have shown reduced abulia, anergia, and anhedonia in those with TBI.34,35 Amantadine may cause confusion, hallucinations, and hypotension. Small studies have suggested some benefits of bromocriptine in cognitive function.36,37 Arousal-enhancing agents also have found a use in the treatment of the neurocognitive sequalae of TBI. Modafinil is the oldest form of these medications, and armodafinil is an isomer of modafinil with longer activity and less side effects. These medications help to increase alertness and wakefulness. The precise mechanism of action of odafinil is unclear. It appears to increase histamine in parts of the brain involved in controlling the sleep-wake cycle; however, knock-out mice that lack histamine receptors still show increased wakefulness with modafinil.38,39 The picture is also murky for modafinil’s effect on orexins, which are wakefulness molecules in the hypothalamus.40 Modafinil has been shown to weakly bind to the dopamine transporter – like the stimulants,41 and dopamine transporter knock-out mice show no response to modafinil.42 A number of research studies have examined the benefit of these agents in fatigue associated with multiple sclerosis, TBI, cancer, and other conditions. Cognitive and memory impairments after TBI may reflect disruption of cholinergic function. The impact of anticholinergic agents on cognitive function of those with TBI supports this contention. Donepezil is the safest and most widely used of the cholinesterase inhibitors. Several easonably large studies have shown improved memory and cognitive function.43-45 Donepezil has benefits in memory and cognition even several years after injury.45,46 Anticonvulsants are often prescribed initially after a TBI due to heightened risk for seizures. Post-TBI mania or mood lability may respond well to anticonvulsants, such as carbamazepine or sodium valproate. They are also often used to treat aggression after TBI. The anticonvulsant agent, topiramate, has been shown to adversely affect cognitive function in the TBI patients.47 While insomnia is a significant issue for patients with TBI, affecting between 15% and 84% (mean of 40%),3,13,19,21,23,48,49 little has been published on the treatment of this aspect of TBI. Benzodiazepines may be effective but carry a risk of disinhibition. Kemp et al48 found that commonly used sleep aid, melatonin, was not effective. Antidepressants, including serotonin reuptake inhibitors and tricyclic antidepressants, are not effective in resolving insomnia in this population.49 No single agent has emerged as a good solution for this symptom. Cognitive rehabilitation Cognitive rehabilitation now takes many forms and is often individualized to the particular needs of the patients. Protocols have been devised to remediate cognitive difficulties often encountered in those with TBI, such as impaired concentration, executive dysfunction, inattention, visual disturbances, memory dysfunction, and impaired language function. They range from simple strategies (using a planner to aid memory and organization) to specific protocols targeting particular cognitive functions (eg, short-term memory) that can be monitored with sequential neuropsychological testing. These interventions have been extensively reviewed elsewhere.50,51 Comprehensive programs which include psychotherapy and social skills components have been shown to have greater efficacy.50,52,53 Overall, reports of benefits have been mixed.54,55 Behavioral therapies Behavioral remediation strategies to eliminate problematic behaviors following TBI have met with mixed success, most often in terms of the poor generalization of specific skills to the outside world. Behavioral deficits that create difficulties for those with TBI and their families include poor hygiene, decline in tidying/cleaning habits, social withdrawal, reduced social comprehension, impaired memory, and poor organization. Behavioral excesses that create difficulties for those with TBI and their families include aggression, sleep disruption, and perseverations. These have been reviewed elsewhere.56 Nutritional supplements Nutritional supplements, herbs, and nootropics have been utilized for many years and are increasingly popular among the patient populations. There remains little clinical research on many of these agents, perhaps reflecting a lack of funding more than a lack of efficacy. Acetyl-l-carnitine is an ester of l-carnitine and is thought to protect brain cells after injury when glucose metabolic pathways are compromised. During this period, acetyll- carnitine supports alternative ketogenic pathways for metabolism.57 It is also believed to enhance cholinergic function. While there are several clinical studies on patients with Alzheimer’s disease and preclinical data on animal models of TBI, the clinical literature on TBI remains sparse. Ginkgo biloba is a natural product of the tree by the same name. It has been shown to improve membrane fluidity and increase resistance to free-radical damage. It provides some subtle benefits to cognitive function in clinical studies of stroke, dementia, aging, and hypoxia damage.58 It has not been systematically studied in TBI but is used extensively in clinic, often in combination with meclofenoxate which is an avid scavenger of free radicals.59 S-Adenosylmethionine (SAMe) is a nutritional supplement which improves cell membrane fluidity and promotes the production of glutathione, an antioxidant. The benefit of SAMe has been assessed in a single clinical study of TBI.60 Patients receiving SAMe had a 77% improvement in clinical scores of post-concussive symptoms. Citicholine provides a source of choline which can cross the bloodbrain barrier. It has been used extensively in Europe and Japan as a treatment for TBI, stroke, and dementia. However, two large US studies failed to demonstrate significant benefit.61,62 Piracetam and the related oxiracetam and phenylpiracetam have shown some promise as nootropic agents. In one double-blind, placebo-controlled study, piracetam improved several symptoms of postconcussive syndrome, including headache and vertigo.63 More recent clinical studies have shown marginal benefit.64 Huperzine-A, an extract of Japanese club moss, is a natural acetylcholinesterase inhibitor. It may serve as a natural alternative to donepezil, rivastigmine, or galantamine. Galantamine warrants special mention as it appears to also modulate nicotinic eceptors and appears to have more persistent benefit in the treatment of Alzheimer’s disease. It appears to modulate neuroimmune responses, in addition to its effects on acetylcholinesterase.65 Cerebrolysin is a polypeptide that purportedly mimics the actions of neurotrophic factors.66,67 Studies have shown that it can reduce beta amyloid and phosphorylated tau protein accumulation. It may promote neurogenesis, synapse formation, and functional recovery.66 In animal models of acute TBI, cerebrolysin-treated rats had more surviving neurons in the area of impact and showed greater functional recovery.67 In a clinical trial of acute TBI, patients were recruited within 24 hours of injury and treated for 3 months with daily intravenous infusion of cerebrolysin. At 3 months, those receiving cerebrolysin performed significantly better on the Cognitive Abilities Screening Instrument.68 It remains unclear if cerebrolysin provides long-term nootropic benefit. The elevation of free radicals in TBI suggests that antioxidants should be beneficial. Clinical trials of pharmacological antioxidants over the past 30 years have not yielded a useful agent in acute TBI.69 Agents, such as tirilazad70 and polyethylene glycol- onjugated superoxide dismutase, have failed to show benefit in acute TBI. Omega-3 fatty acids may enhance brain repair and recovery, based on animal and clinical studies.71 Similarly, vitamin D may offer neuroprotective and restorative benefits72 in the acute TBI setting. In chronic TBI, vitamin D and omega-3 fatty acids may work synergistically, as they both may reduce neuroinflammation, apoptosis, and oxidative stress.73 Other nutritional supplements have been recommended, but prolonged therapy is necessary to possibly see benefits in TBI. A 6-month trial of ginkgo, vinpocetine, acetyl-lcarnitine, huperzine, alpha-lipoic acid, n-acetyl-cysteine, multivitamins, and over 5 g of omega-3 fatty acids daily yielded improved performance in cognitive testing and increased perfusion (function) in single-photon emission computed tomography (SPECT) scan.74 Long-term use of dietary flavanols may improve cognition in mTBI.75 Transcranial magnetic stimulation Transcranial magnetic stimulation (TMS) has shown some promise in animal models of TBI.76 However, a Cochrane review of the clinical application of TMS for depression noted no difference between repetitive TMS (rTMS) and sham rTMS using the Beck Depression Inventory (BDI) or the Hamilton Depression Rating Scale, except during the initial 2-week period.77 The application of TMS in the post-TBI patients is limited by the risk of seizure induction.78 Hyperbaric oxygen Hyperbaric oxygen treatment has been explored as a treatment for TBI.79-91 Hyperbaric oxygen therapy is neither a benign treatment, given the concerns of oxygen toxicity,79 nor a clear treatment in that the placebo condition of moderate hyperbaric room air also effectively improves cognitive function.80,81 The most carefully performed study compared a group in a cross-over design with an interval of both null treatment and hyperbaric oxygen at 100% oxygen and 1.5 atm.82 The study described improvement in many of the symptoms associated with persistent TBI including headache, tinnitus, vision disturbance, memory dysfunction, and impaired cognitive function. Cognitive testing also showed improvement in attention, information processing speed, and a battery of cognitive tests. In an uncontrolled case series of 16 subjects, Harch et al83 demonstrated that an abbreviated series of hyperbaric treatments using 100% oxygen at 1.5 atm could mitigate subjective symptoms of TBI (eg, headache, sleep disruption, irritability), improve cognitive testing scores, and improve cortical function based on SPECT imaging.83 A study of a higher dose (2.4 atm) did not reveal any significant benefit of hyperbaric oxygen therapy compared to a sham-control group treated with 1.3 atm,84 and this result has been extended and confirmed by a related group.85 However, this may reflect an inverse dose- esponse curve, rather than an absence of benefit, in that the low-dose sham group demonstrated significant changes in cognitive testing and symptom frequency.86 Hyperbaric oxygen remains a controversial area in both acute TBI86-89 and chronic TBI.82,83,85,86,90,91 Physical exercise High-energy activities and exercise programs completed through a health club facility or comprehensive rehabilitation program should focus on the same parameters of an age-adjusted and diagnosis-specific program for aerobic conditioning – flexibility, stabilization, and strength. Though it appears safe and is an accepted intervention for TBI, there is a need for further welldesigned studies.92 Exercise was a part of a 6-month study of lifestyle changes described above which yielded improved function based on cognitive testing and perfusion SPECT scans.74
A NEW TREATMENT FOR TBI
Unfortunately, little has been found to reverse the damage of TBI or repetitive concussion which is the root cause of residual cognitive and psychological impairment following TBI.20,93 One potential avenue of treatment for TBI is infrared light, which has shown promising data in a number of applications. Near-infrared (NIR) light has been investigated for its ability to modulate intracellular mechanisms related to healing. The application of NIR light by low-power laser or by light-emitting diode (LED) is also known as laser phototherapy94 or near-infrared photobiomodulation.92 NIR irradiation can facilitate wound healing,95,96 promote muscle repair,95 and stimulate angiogenesis.95,96 NIR phototherapy has been studied and applied clinically in a wide array of ailments, including skin ulcers,97 osteoarthritis,98 peripheral nerve injury,95,96 low back pain,99 myocardial infarction,100 and stem cell induction.101 The finding that NIR light passes relatively efficiently through bone has spurred interest in its application to treating disorders of the brain. Over the past decade, transcranial near-infrared light therapy (NILT)102 has been studied in animal models to understand its ability to repair damaged or dysfunctional brain tissue resulting from stroke and TBI. The first published study of NILT for TBI in humans described two cases of chronic mTBI with significant disability.103 Each patient was treated with an LED device delivering low-level low-level light therapy (LLLT) in the red and NIR range for 6-10 minutes per area daily for several months. Both patients had marked neuropsychological improvement after a minimum of 7-9 months of LLLT treatment. The precise mechanisms underlying photobiomodulation and its therapeutic benefits are not fully understood. The purported effects of NIR are illustrated in Figure 1. Light in the wavelength range of 600-1,200 nm has significant photobiomodulation capability.104 Current data most strongly support that absorption of NIR photons by cytochrome c oxidase in the mitochondrial respiratory chain is the key initiating event in photobiomodulation.95,96,104,105 This induces an increase in cytochrome c oxidase activity which in turn increases adenosine triphosphate (ATP) production. Such an increase in ATP in wounded or underperfused cells may be sufficient to activate cells in areas of injury or metabolic derangement.106 Data from numerous tissue culture and animal studies point to the importance of several secondary molecular and cellular events. For example, NIR photonic energy can modulate reactive oxygen species,95,96,102 activate mitochondrial DNA replication,95,96 increase early-response genes,95 increase growth factor expression, induce cell proliferation, and alter nitric oxide levels.95,96,102 These mechanisms are more fully described in the companion paper.105 When examined in the specific model of neural tissue injury, NIR phototherapy can lead to demonstrable neural repair and recovery. For example, LLLT of a power density of 0.9-36 J/cm2 applied at 24 hours poststroke in a rodent model yielded a 32% reduction in neurological deficits, as well as histochemical evidence of neuron proliferation and migration.106-108 LLLT had similar benefits in a rodent model of TBI.96,109-111 Interestingly, these cellular changes evolved over a period of days after light exposure and persisted for considerably longer than the interval of actual NIR exposure. These findings are consistent with a progressive regeneration cascade set in motion by the NIR light exposure. NILT in stroke NILT, predominately in the form of LLLT, has been investigated in laboratory models of stroke. LLLT applied in a single dose to an ischemic stroke model appeared to induce expression of the growth factor transforming growth factor – beta 1 and suppress the production of peroxynitrite.112 In a rat model of middle cerebral artery occlusion, LLLT at a dose of 0.5-7.5 mW/ cm2 using continuous wavelength light at 808 nm was administered at 24 hours after the acute stroke.108,113 This single application was estimated to deliver 1.8 J/cm2 in total to the cortex surface and resulted in demonstrable neurological improvement. Functional changes were not manifested until approximately 2 weeks after the single treatment. While there was no significant change in the size of the stroke lesion, histochemical evidence of neurogenesis and migrating neurons108 indicate that a cascade of secondary processes was initiated by NILT. A rabbit model of stroke utilizing injection of a blood clot embolus also demonstrated benefit from LLLT.102,114,115 Herein, 808 nm light was applied with an LED delivering 7.5 mW/cm2 and an estimated 0.9-2.6 J/cm2 to the cortical surface. Cortical ATP levels were increased, indicative of increased mitochondrial activity.114 Significant behavioral recovery was also noted; however, neither ATP increased nor neurological function changed at doses less than 0.3-0.7 J/cm2.114,115 At higher doses of 0.9-15 J/cm2, neurological improvement was seen.114,115 The clinical trials of NILT in acute stroke, the Neuro- Thera Effectiveness and Safety Trials 1, 2, and 3 (NEST- 1,-2, -3), were conducted between 2006 and 2009. The Phase II clinical trial (NEST-1) involved 120 patients in a double-blind, placebo- ontrolled study of the effects of NILT within 24 hours of ischemic stroke.116,117 Approximately 60% of the patients experienced clinical benefit, and the safety profile was very good. Thus, NEST-2, a Phase III clinical trial, was undertaken in 2007. A total of 660 patients were enrolled.118 Somewhat surprisingly, the study did not demonstrate statistical clinical improvement using a different outcome measure.119 Post hoc analysis revealed that a portion of the patients who were moderately affected and/or had strokes limited to the cerebral cortex did realize clinically and statistically significant improvement.102 The NEST-3 trial was halted midpoint when it failed to demonstrate statistical benefit on futility analysis.120 A key factor in the interpretation of the results of NEST-3 is that, different from NEST-1, all types of stroke were included as opposed to just cortical strokes. Continuous laser light has a limited depth of penetration (#1 cm into brain tissue) which likely prevents an effect on deeper brain matter. Therefore, the lack of significant benefits from NIR phototherapy in NEST-3 could be related to the fact that ischemic penumbra was not reached by the light (Luis DeTaboada, personal communication, January 2015). While pulsed NIR was not used in the NEST-3 study, it is estimated that pulsed NIR could penetrate up to 3 cm in depth from the cortical surface, therefore possibly extending the therapeutic target to deeper strokes (Luis DeTaboada, personal communication, January 2015). Figure 1 Hypothesized mechanism of action of NiR light therapy. Notes: NiR light (600-980 nm) penetrates tissue to variable depths depending on wavelength, the tissue involved, coherence, and time. A fraction of the photonic energy reaches the mitochondria and is absorbed by cytochrome c oxidase. This activates increased ATP production, increases production of ROS and RNS, and possibly increases NO. Downstream events include increased early-response genes (c-fos and c-jun) and activation of NF-?B, which in turn induces increased transcription of gene products leading to synaptogenesis, neurogenesis, and increased production of inflammatory mediators and growth factors. Abbreviations: NiR, near-infrared; ATP, adenosine triphosphate; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide; NF-?B, nuclear factor kappa B. NILT in TBi Oron et al109 conducted the first animal studies of NILT for TBI. They found that a single application of NIR light at 808 nm from a 200 mW emitter at 4 hours post-injury resulted in a significant reduction in lesion size by 5 days.109 To date, several groups have studied NILT in animal models, and this material has previously been reviewed.95,121-123 Single applications of 800-810 nm NIR light within 4 hours of injury have been shown to improve neurological function significantly.110,124-126 The same dose of NIR light at 6 hours was less effective125 and at 8 hours had no appreciable benefit.125 NIR photonic energy at other wavelengths was less effective. Wu et al110 examined red light (670 nm) at 4 hours and found a similar improvement in neurological function; however, 730 nm and 980 nm had no neurological benefit. Similar data for lesion volume have been reported. A single dose of 800-810 nm NIR light (fluence of 36 J/cm2) yielded an approximate 50% reduction in the volume of the lesion at 3-4 weeks110,111,124-126 and a possible reduction in the initial spread of neurological injury, based on the marked reduction in lesion volume found at 5 days post-injury.109 Repeated NIR phototherapy treatments appear to have some benefit, but the frequency and number of treatments are critical factors. While a single NIR light application had benefit, daily applications for 3 days yielded much greater neurological benefit126,127 with smaller lesion size,126 fewer degenerating neurons,126 more proliferating cells,126 and greater levels of brain-derived neurotrophic factor (BDNF)127 compared to a single treatment in a mouse model. In contrast, daily treatment for 7 days128 or 14 days126 showed no difference from controls. NIR energy densities in the range of 0.9-36 J/cm2 resulted in significant biochemical and behavioral changes.109-111,124-127 Pulsing of NIR light appears to yield a greater neurological response but only within certain parameters. Pulsing at 10 Hz yielded greater neurological improvement and a significant reduction in lesion size compared to either continuous-wave or pulsed NIR at 100 Hz.111 In the mouse model of moderate TBI, NILT (800-810 nm) improved learning and memory (Morris water maze performance),128 as well as behaviors associated with depression and anxiety (immobility during tail suspension).111,124 The finding that NILT brought about a smaller lesion in the rodent model of TBI compared to untreated mice suggests that decreased apoptosis, reduced spreading lesion penumbra, and/or neurogenesis are induced by NILT. Indeed, NILT can decrease BAX expression, a pro-apoptosis gene,129 increase expression of BCL-2, an anti-apoptosis gene,129 increase nerve growth factor,95 increase BDNF,127 decrease inflammatory markers,130 and decrease numbers of degenerating neurons.126 Together, these mechanisms may reduce the enlargement of the initial lesion during the first day following the lesion.109 Moreover, increased BDNF and nerve growth factor may contribute to synaptogenesis as shown by increased levels of synapsin-1,127 and neurogenesis, as shown by increased numbers of proliferating cells.127 In a double-blind study in healthy volunteers, NILT was beneficial – compared to sham – in memory and attention.131 In this study, the authors shed only one application of NIR light to the right forehead, targeting the right frontal pole of the cerebral cortex (Brodmann’s area 9 and 10). The device was a Class IV laser CG-5000 (Cell Gen Therapeutics, Dallas, TX, USA), and the parameters were as follows: wavelength 1,064 nm, irradiance 250 mW/cm2, fluence 60 J/cm2, and time 4 minutes per site (two sites).131 The subjects who received the NIR treatment had better attention after 2 weeks, measured by the psychomotor vigilance test. They also had better delayed visual memory at the Delayed Match-to-Sample test. This is the only published controlled trial assessing the impact of NILT on cognition; however, other reports have shown the therapeutic effects of NILT in small numbers of TBI patients. In a two-case report in TBI patients,103 NILT (870 nm) improved sustained attention, memory, and executive functions. Both patients were treated with an instrument with three separate LED cluster heads. The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 2.2-25.8 mW/cm2, fluence 13.3 J/cm2, and time 10 minutes per site.103 The same group reported on a cohort of eleven subjects with persistent cognitive dysfunction and treated with a similar NILT protocol for chronic mTBI.132 The eleven subjects received NILT with a device with three LED cluster heads (Model 1100; MedX Health, Toronto, ON, Canada). The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 22.2 mW/cm2, fluence 13 J/cm2, and approximate time 10 minutes per site. The NIR light was applied three times per week for 6 weeks (18 sessions), on eleven sites for 10 minutes per site (the total duration of each session was 20 minutes).132 The sites on the skull were chosen on the midline, and bilaterally on frontal, parietal, and temporal areas. At the follow-up neuropsychological testing, NILT had a powerful effect on attention, inhibition, and inhibition switching in the Stroop task, and similarly improved verbal learning and memory, as well as enhanced long-delay free recall on the California Verbal Learning Test. Eight subjects, from the same cohort, were identified as having mild, moderate, or severe depression based on the BDI-II total score (range: 15-34).132 The three cases, who entered the study with only mild depression, remained the same after NILT treatment. Results for the five cases with moderate-severe depression were as follows: two moderate cases improved to mild/minimal depression 8 weeks after the end of NILT series, and one severe case improved to moderate depression. Two moderate or severe depression cases remained the same after 8 weeks of follow-up from the last NILT session.132 Dose response and photonic penetration A prevailing theory in photobiomodulation postulates that a bimodal response curve exists for the biological effects of NIR light.95 The so-called Arndt-Schulz curve (a fundamental principle in homeopathic medicine) is frequently used to describe this biphasic dose response. Some data indicate that low levels of light have a much better effect on stimulating and repairing tissues than higher levels of light. Laboratory studies of cells in culture have demonstrated a bimodal dose response to light exposure in lymphocytes133 and fibroblasts.134,135 For example, Chen et al135 found that a range of 0.03-0.3 J/cm2 was beneficial in activating transcription factors in culture, while 3-30 J/cm2 inhibited the activation of these factors. In contrast, an order-of-magnitude greater dose (2 J/cm2) was best at activating fibroblasts in a superficial wound model.136 Furthermore, an order-ofmagnitude greater dose (30 J/cm2) proved to be best in a rodent joint inflammation model.137 Thus, a dosedependent effect for many biological responses to NIR light has been demonstrated,95,137-139 but the critical parameter is dose at the level of the target tissue, rather than at the surface.137,140 The amount of energy that reaches a volume of tissue at depth is determined by the attenuation of the photonic energy as it passes through the overlying tissue. For example, only 2.45% of the energy from a 980 nm laser emitter penetrates to the level of the peroneal nerve.140 Nevertheless, the biphasic dose response does not appear to be universally true. In primary microglial cell culture, a dose-dependent response to NIR was demonstrated with no detrimental effects at doses as high as 30 J/cm2.141 So a critical question in the use of NILT is that of radiant energy penetration. In particular, some authors have challenged the efficacy of low-power LEDs used in LLLT.142-144 In laboratory studies, LLLT radiant energy is almost entirely absorbed in the first 1 mm of skin.145,146 In two unrelated studies, LLLT diode devices proved to be ineffective in the treatment of diabetic neuropathy,142,144 in contrast with prior reports.147 Similarly, laboratory studies of NILT using LLLT transcranially have not consistently yielded positive results. For example, in a rat model of TBI, Giacci et al148 found no benefit from daily 30-minute irradiation with either 670 nm or 830 nm 0.5 W LED emitters for a period of 7 days. Doses at the skin surface were 28.4 J/cm2 and 22.6 J/cm2, respectively.148 Similarly, treatment of a rat model of contusive spinal cord injury with LLLT (830 nm at 22.6 J/cm2 or 670 nm at 28.4 J/cm2) for 30 minutes per day for 5 days resulted in no significant functional improvement and no reduction in lesion size, despite delivering 2.6 J/cm2 to the spinal cord.148 Lapchak102 reported that the physical parameters of NILT in the clinical trials for the treatment of stroke utilized in the NEST-1 and NEST-2 trials116-120 may have delivered insufficient energy to cortical tissues to be effective. Therein, NIR light of 808 nm wavelength with infrared energy densities of 0.9 J/cm2 was applied to the human scalp for a total of 40 minutes with applications at multiple sites during that time.116,118 Recall that animal models of both stroke and TBI suggest that NIR energy densities in the range of 0.9-36 J/cm2 resulted in significant biochemical and behavioral changes.96,106-115,125-127 The concern raised from the NEST studies102 is that current clinical trials testing the effectiveness of lowenergy NIR diodes to treat TBI may yield negative or inaccurate efficacy data, not because of a failure of infrared light to invoke a change but due to a dose error. Doses that are effective when directly applied to cells in a Petri dish149,150 or to 3-5 mm thick rodent brains96,109-111,125,126,128 may be insufficient to penetrate 2-4 cm into the human brain. In a companion paper, our own studies of photonic energy penetration are detailed.105 To summarize, the laboratory tissue studies showed that 0.5 W LED emitters did not penetrate the 2 mm thickness of human skin. No detectable energy from 0.5 W LED NIR light emitters could be detected penetrating a similar thickness (1-2 mm) of sheep skin or 3 cm thick section containing sheep skin, skull, and brain. In contrast, 11% of the photonic energy from a 10 W 810/980 nm coherent NIR laser penetrated 2 mm of human skin. Similarly, 17% of the photonic energy from a 15 W 810 nm coherent NIR laser penetrated the same distance.105 Energy from these more powerful NIR emitters could be detected penetrating 3 cm of sheep skin, skull, and brain with 0.4% of the 10 W 810/980 nm NIR laser’s energy reaching the depth of 3 cm and 2.9% of the 15 W 810 nm NIR laser’s energy traversing the same distance.105 Anders also has demonstrated penetration of 808 nm light to 40 mm in the brain using a 5 W laser emitter (JJ Anders, personal communication, January 2015). Prompted by the mixed results in the literature and the observations by Lapchak,102 Franzen-Korzendorfer et al,144 Wan et al151 and Lavery et al142 we have been utilizing relatively high-power (10- 5 W) lasers at the wavelengths of 810 nm and 980 nm in the clinic to treat patients with TBI. Clinically, the patients have shown excellent responses with resolution of many of their long-standing symptoms of TBI or post-concussive syndrome. Below is a retrospective series of such patients to illustrate the extent and character of response to this modality. Methods Patients in the case series were sequentially treated patients at a clinic which is engaged in ongoing NILT for a number of clinical conditions. The risks, benefits, and current state of research on the use of NILT were explained to each patient. Each patient consented to treatment. Institutional Review Board approval was obtained in a post hoc review, noting that the risk-benefit ratio was acceptable. Between March 16, 2011 and February 20, 2013, sequential new referrals for chronic mild-to-moderate TBI were evaluated for treatment and selected for NILT using Class IV lasers, either the LT1000 (LiteCure, Newark, DE, USA), a 10 W adjustable NIR laser emitter with wavelengths of 810/980 nm capable of delivering continuous or pulsed NIR light, or the Diowave 810 (Diowave, Riviera Beach, FL, USA), an adjustable NIR emitter up to 15 W with a wavelength of 810 nm capable of delivering continuous or pulsed NIR energy. Demographics and laser treatment settings are detailed in Table 1. The fluence delivered to the skin of patients ranged from 55 J/cm2 to 81 J/cm2. No other treatment modalities (medications, exercise regimen, supplements) were added, discontinued, or changed while receiving NILT. Symptoms were monitored clinically. A baseline Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)152 was completed for all patients, and the BDI153 was administered to seven of the ten patients before and after the course of treatment. In addition, each patient was instructed on how to create and maintain a patient and spousal diary of symptoms and subjective progress. Each of six patients received a single series of ten treatments with the LT1000 Class IV laser. Three additional patients each received a single series of 20 treatments with the LT1000 Class IV laser. One patient was treated with the Diowave 810 nm Class IV laser device in a series of 20 treatments. The patients and treating clinician wore protective eyewear. There were no incidents of burns or thermal discomfort (Figure 2). The impact of high-watt NILT While the patient group represented a diverse mix (Table 1 presents demographics), some notable commonalities of symptoms emerged. Over 90% of the patients had complaints of anxiety, depression, irritability, and insomnia. Other symptoms included headache (60%), suicidal ideation (50%), cognitive difficulties (50%), attention problems (50%), short-term memory problems (40%), loss of libido (30%), substance abuse (20%), fatigue (20%), and panic attacks (20%). Six of the patients were unemployed prior to treatment. Three of the patients were experiencing severe marital difficulties. All carried or had a confirmed diagnosis of TBI, but other comorbid diagnoses included PTSD, major depressive disorder, generalized anxiety disorder, bipolar disorder, and attention deficit/hyperactivity disorder. The patients’ baseline scores on the BDI were 25.3±12.1 (moderate depression range), and baseline scores on the QIDS-SR were 12.9±4.6 (moderate depression range). During NILT treatments, skin temperature increased no more than 3°C with rapid cooling after removal of the NIR light. A continuous sweeping motion was utilized to minimize skin heating and cover a larger area. After a course of ten treatments of NILT (20 treatments in four patients), each patient experienced significant clinical improvement with resolution of many of their symptoms (Table 2). In addition, the BDI scores dropped to 12±6.5 (nondepressed range). This represented a significant decrease (P,0.01, Student’s t-test, one-tailed, Microsoft Excel). The QIDS-SR scores after treatment were 2.2±2.3 (nondepressed range), and the difference from baseline was highly significant (P,0.00001, Student’s t-test, one-tailed). Patients noted improvement in cognitive function, mood, anxiety, and sleep. None of the patients continued to have suicidal thoughts (50% at baseline). Other symptoms, such as anxiety and irritability, were markedly improved. Most notable were the nonquantifiable changes in patients’ lives. Patients reported improved cognitive ability and a desire to return to meaningful work. Five of the six unemployed patients have returned to work. The two patients who were Iraq/ Afghanistan veterans have found new careers in highly skilled trades. The patients with marital difficulties have reconciled and were purchasing homes or otherwise solidifying their marriages. The clinical change can be attributed to NILT because no changes in medications, supplements, or exercise regimen were permitted during the course of NILT treatment. All patients in the case series experienced significant clinical improvement which supports the conjecture that high-power NIR laser delivers sufficient energy to the human brain for photobiomodulation to occur. Insomnia and suicidal ideation, common symptoms in those with TBI or post-concussive syndromes,3,17-20,24,25 resolved in 100% of cases. Headache, another common symptom for patients following a TBI,6,14,15,23 was reduced or resolved in the six patients so afflicted. Symptoms such as anxiety,14,15,21,24 depression,21,24,25,27-29 and irritability resolved or were dramatically reduced in all patients. Cognitive function appeared to improve based on return to work or improved work performance, although cognitive tests were not performed. The quality of life dramatically improved in all cases, based on the observations of the patients, their family members, and the treating clinician. At follow-up intervals of 6-7 months post-treatment, patients have reported continued improvements in symptoms. The precise areas of brain injury were not elucidated in Figure 2 Treatment parameters per individual, based on area of the skull treated. Notes: Dimensions varied per head/skull size and hair line. Treatment was warm and comfortable for each patient. There were no incidences of discomfort. Areas treated were (A) temporal- ilateral, (B) frontal, and in patients 1-3, 5, and 6 (B) frontal only. Table 1: Infrared light treatment parameters for each of the ten patients in the case series Patient Area treated Sex Mechanism of TBI Interval since TBI Wavelength of Dosage per area Duration before treatment NIR-PT dual wave Scanning technique per area pulsed 10 Hz 1 B, bilateral frontal Male Concussive blast 2 years 810 and 980 nm 2,700 J 10 minutes Fluence – 20.45 J/cm2 2 areas Area – 132 cm2 10 visits 2 B, bilateral frontal Female MVA 18 years 810 and 980 nm 2,400 J 9 minutes Fluence – 18 J/cm2 2 areas Area – 133 cm2 10 visits 3 B, bilateral frontal Female MVA 5 years 810 and 980 nm 2,400 J 8 minutes Abuse Fluence – 18.3 J/ cm2 2 areas Area – 131 cm2 10 visits 4 A–B, bilateral frontal, left temporal Female MVA x2 8 years and 13 years 810 and 980 nm 2,400 J 8 minutes Fluence – 18.3 J/cm2 3 areas Area – 131 cm2 10 visits 5 B, bilateral frontal Male Vietnam Veteran 20+ years 810 and 980 nm 3,000 J 10 minutes Concussion Fluence – 28.3 J/cm2 2 areas Child abuse Area – 106 cm2 10 visits 6 B, bilateral frontal Male Concussion 5+ years 810 and 980 nm 2,400 J 12 minutes Fluence – 14.8 J/cm2 2 areas Area – 162 cm2 10 visits 7 B–A, bilateral frontal, left temporal Male Afghanistan, Iraqi Disability 810 and 980 nm 3,000 J 10 minutes Disability due to TBI 2 years Fluence – 22.7 J/cm2 3 areas Area – 132 cm2 20 visits B–A, bilateral frontal, bilateral temporal Female Hypoxic encephalopathy Childbirth-related 810 and 980 nm 2,700 J 9 minutes injury, 8 years Fluence – 27.8 J/cm2 3 areas Area – 97 cm2 20 visits 9 B–A, bilateral frontal, bilateral temporal Male MVA-TBI Numerous episodes 810 and 980 nm 3,000 J 10 minutes Concussions Fluence – 22.72 J/cm2 3 areas Area – 132 cm2 20 visits 10 B–A, bilateral frontal, left temporal Female Bicycle vs car >30 days 810 nm single 2,700 J 9 minutes Concussion, amnesia, LOC wavelength – Fluence – 17.1 J/cm2 3 areas different device Area – 158 cm2 20 visits Note: All safety precautions were followed, including metal protective eyewear (laser eye protection). Abbreviation: LOC, loss of consciousness; MvA, motor vehicle accident; TBi, traumatic brain injury. the majority of these cases, so a correlation of symptoms changes and cortical function changes cannot be made; however, perfusion SPECT imaging in other patients has shown significant increases in perfusion in injured areas of the brain and overall improved cortical function following similar courses of high-watt NILT.154 One concern that has been expressed about high-watt NIR lasers is the risk of tissue heating.155 We explored this issue in our companion paper on NIR penetration.105 Temperature change was 1°C-3°C at the skin surface using continuous-wave NIR lasers in the range of 10-15 W. Using pulsed settings, the high-powered lasers showed no significant temperature change in tissue samples. The temperature change on human skin was 1°C or less in the in vivo penetration studies while maintaining continuous movement of the laser probe head.105 Clinically, patients in this case series reported only slight warming of the skin, but no discomfort, using the continuous motion technique. Laboratory studies have largely focused on treatment of acute brain injury. The processes involved in the benefits of NIR light in chronic TBI as seen in this clinical case series may be quite distinct. Nevertheless, Schiffer et al156 found that a single application of LLLT at 810 nm and 250 mW to the forehead over 8 minutes reduced depression and anxiety symptoms in ten patients for approximately 2 weeks. Similarly, the small case series by Naeser et al103 demonstrated some benefit using NIR light, albeit at very low power levels over a prolonged course of several months with only transient benefit. Together with our clinical data, these findings suggest that at least some of the photobiomodulatory effects of NIR energy likely do occur in chronic neurological conditions. Prior presentations on NILT for the treatment of TBI or stroke in humans have focused on getting photonic energy through the skull to the cortex surface which traverses a distance of about 6-10 mm; however, this model is flawed in that the distance to the areas of damage may be far greater. In other words, the cortex immediately subjacent to a portion of the skull may be 10 mm from the surface, but the NIR light energy may need to penetrate 3-7 cm to reach areas of damage. Much of the cortical surface is actually lining the walls and floors of sulci, rather than immediately subjacent to the skull. Analysis of NIR spectroscopy reveals that light propagation through varying media with irregular boundaries is subject to high levels of scatter.157 In addition, review of the neuroimaging literature on TBI has revealed that the most common areas injured in TBI are the orbitofrontal cortex (at the ventral surface of the frontal lobe) and the anterior and medial temporal lobes.158 It is not anatomically possible to position an NIR light emitter immediately exterior to the skull overlying these areas. Indeed, the orbitofrontal cortex positioned immediately above the eyes can only be reached from the forehead by angling the light emitter. Similarly, the temporal lobes are separated from the surface by epidermis, dermis, subcutaneous fat, subcutaneous blood vessels, accessory head of the temporalis muscle, connective tissue, temporalis muscle, skull, and dura mater.159 Each of these structures has different absorption and refraction properties, and each interface between different materials also creates a barrier to transmission of photonic energy.157 Blood flowing in the subcutaneous vessels is believed to create a unique barrier to transmission.160 In summary, effectively targeting the areas most commonly injured in TBI with sufficient photonic energy to initiate reparative processes represents a significant challenge in NILT. This appears to have been overcome with the high-power laser protocol presented here and in a related paper.154 As yet, the mechanism of action of NILT in treating TBI is not entirely clear. Moreover, the neurological benefits are not immediately apparent. Rather, a delay of 1-4 weeks was noted, consistent with a progressive regeneration cascade set in motion by the NILT.96,103,105 ,107,109,121,122,124,127,135 Similarly, most of the patients in the present case series did not notice benefits immediately or within the first few treatments. Instead, they reported benefits emerging over an interval of weeks, and in some cases, continuing after completion of the course of NILT. In addition, the clinical improvement reported by the patients in the above case series is more profound than that reported by patients treated with LLLT or low-powered lasers.103 In fact, we observed that among seven subjects with documented moderate depression, per BDI scores, four had an antidepressant response (≥50% decrease of depression severity). In contrast, Naeser et al132 reported that out of eight subjects with TBI and comorbid depression, only three had a significant improvement in their depressive symptoms (37.5%). Our results may be due to the greater penetration of more powerful, coherent, and pulsed NIR light from a laser source. A unique outcome measure was developed for this protocol (Morries and Henderson, unpublished data, 2015). A patient diary and separate spousal diary provided a weekly update of patient’s response in his or her home environment. This novel approach to capturing the patient treatment experience provided the patient and family with tangible and pertinent documentation of the clinical response. While time consuming, the experiences recorded in these diaries proved to be valuable clinical tools to the treating clinicians.
CONCLUSION
To date, there has been little progress in developing effective treatments for chronic mild-to-moderate TBI or repetitive concussions. This area of need has become even more pressing with the return of veterans from military conflicts in Iraq and Afghanistan4,6,7,16,17,19,161 and the recognition of the magnitude of sport-related TBI.5,8-10 In addition, the dramatic growth in the geriatric population with attendant proprioceptive dysfunction has resulted in a rising incidence of fall-related TBI.162 NILT has shown promise as a tool for the treatment of TBI. A critical issue is to assure that adequate photonic energy reaches the injured areas of the brain. The use of high-wattage lasers, as we have demonstrated, results in marked clinical improvement in patients with chronic TBI. Moreover, symptoms consistent with PTSD, anxiety, and/or depression also improved considerably or resolved in this group of patients. Further work in the use of highwattage NILT in the treatment of TBI, depression, and other neurological disorders is encouraged.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Mr Charles Vorwaller (Aspen Lasers) and Lite Cure Corporation. The authors also acknowledge the contribution of Ms. Taylor Tuteur in the artistic creation of Figure 1.
DISCLOSURE
Dr. Larry D Morries is the CEO of Neuro-Laser Foundation, a nonprofit foundation. He has a private practice in Lakewood, CO. Theodore A Henderson is the president of The Synaptic Space, a medical consulting firm. He is Table 2 NiLT case series with demographics, symptoms, and treatment response
PRETREATMENT POSTTREATMENT
Patient # Sex Occupation Mechanism of TBI Diagnoses Sleep Symptoms Suicidal BDI Sleep Symptoms Suicidal BDI 1 M Veteran, Blast – 5 years; TBI, PTSD, MDD Primary and H, S, I, D, X, L, A, M, + – Resolved None, back No – unemployed Iraqi middle C, SL with spouse, insomnia working 2 F Nurse, MVA – 8 years TBI, PTSD Middle and H, F, I, X, C, A, STM, L, + 18 Resolved A and HA – No 15 unemployed terminal HA, SL but mild, insomnia return to work 3 F Unemployed Assault and TBI, PTSD, MDD, Primary and D, X, P, M, L, HA, S, + 23 Resolved HA – mild, No – MVA, 5 years GAD, ADHD middle insomnia, SA, C, N, STM back with Prior nightmares spouse, no SA, working 4 F Unemployed MVA – 3 years, TBI, PTSD, MDD Primary and D, X, HA, I, M, SA, S, N + 23 Resolved None, marriage No 17 assault middle insomnia, improved, numerous violent nightmares no SA, working 5 M Veteran, Blast – 20+ years TBI, MDD, GAD Primary and D, X, I, S, SL + 18 Resolved None No 1 unemployed 1960s; Vietnam middle insomnia 6 M executive Trauma – TBI, GAD, MDD Primary D, X, I, P, HA, A, S – – Resolved HA, X, and P – No – chronic insomnia but improved 7 M Veteran, Multiple blasts TBI, MDD, GAD Primary and S, D, I, X, C, A, S, STM, – 22 Resolved HA and C – No 16 disability (>12); Afghan middle HA mild, new and Iraqi wars insomnia career 8 F Student Childbirth TBI, learning Primary D, I, X, C, A, SL, F, STM – 16 Resolved, STM improved, No 7 disorder insomnia no bads reading .20% dream more animated 9 F Sales MVA and TBI, LOC Primary and HA, SL, N, D, I, X, H, A – 29 Resolved Mild HA, No 9 sports TBI middle insomnia, job nightmares promotion 10 F Physicist Recent car– TBI, LOC, amnesia Primary and D, I, X, neck, knee pain – 51 Resolved No loss No 19 bicycle middle of skills, accident insomnia maintain intellectual job Notes: Demographics for each of the ten patients in this case study is presented. Also presented is their history of mechanism of injury, diagnosis, and related symptoms. Changes in anxiety levels, sleep patterns, depression, and suicidal ideation were important symptoms and outcomes to track. Patients were instructed for no medication changes, with their primary treatment provider’s approval. Cognitive difficulties, attention problems, and short-term memory difficulties were by patient interpretation of their symptomatic improvement and patient diary changes. Symptom occurrence % was as follows: Anxiety – 100%, Depression – 90%, Irritability – 90%, Primary And Middle Insomnia – 90%, Headache – 60%, Sadness – 60%, Suicidal Ideation – 50%, Cognitive Difficulties – 50%, Attention Problems – 50%, Short-Term Memory Problems – 40%, Marital Difficulties – 30%, Loss Of Libido – 30%, Substance Abuse – 20%, Fatigue – 20%, Panic Attacks – 20%. Abbreviations: NILT: Near-Infrared Light Therapy, TBI: Traumatic Brain Injury, PTSD: Post-traumatic Stress Disorder, MDD: Major Depressive Disorder, GAD: General Anxiety Disorder, ADHD: Attention Deficit/Hyperactivity Disorder, H: Hyperarousal, S: Sadness, I: Irritability, D: Depression, X: Anxiety, L: Loss Of Libido, A: Attention Problems, M: Marital Difficulties, C: Cognitive Problems, SL: Sleep Issues, F: Fatigue, STM: Short- erm Memory Problems, HA: Headache, P: Panic Attacks, SA: Substance Abuse, N: Nightmares, BDI: Beck Depression Inventory, LOC: Loss of Consciousness, MVA: Motor Vehicle Accident. the president of Dr. Theodore Henderson, Inc., a clinical service firm. He is the co-owner of Neuro-Luminance, a clinical service organization. He is the president of the International Society of Applied Neuroimaging. He is the CFO of the Neuro-Laser Foundation, a nonprofit foundation. Dr. Paolo Cassano received funding from the Brain and Behavior Research Foundation; Photothera Inc and from the Dupont Warren Fellowship (Harvard Medical School) to conduct research on NIR light for the treatment of major depressive disorder.
ABOUT THE AUTHORS:
Larry D. Morries, DC brings a distinguished 30-year career studying and treating the brain and body through his private practice based in Lakewood, Colorado. As Neuro-Laser Foundation’s co-founder, his chiropractic expertise is complemented with extensive study of near infrared-light therapy applications, clinical radiology, clinical neurology and sports injury and rehabilitation. In practice since 1973, Dr. Morries has contributed extensively to both chiropractic and medical professions throughout his career. He is a recognized expert often called upon for review services, treatment utilizations, and documentation presentations. In recent years, he has guided the Colorado State of Colorado Workers Compensation Board with a review of treatment guidelines for Chronic Pain, and Complex Regional Pain Syndrome, Shoulder Pain, Low Back Pain, Traumatic Brain Injury, and was asked to present in 2016 on Thoracic Outlet Syndrome.
Other professional involvement include:
• Colorado Chiropractic Association, Board member, President in 1982, Chairman in 1984
• Colorado Chiropractic Society, Vice President and Secretary in 1995-2004
• Colorado Chiropractic Journal Club, Chairman,since 2008
Dr. Morries has continued his study of the human body and brain with postgraduate work in Neurodiagnostic testing at the American Academy of Neurology, and Harvard Medical School-Massachusetts General Hospital. He is also educated on Spinal Mechanics at Chicago Rehabilitation Institute. He earned his Doctorate in Chiropractic from Logan Chiropractic College, with recognition as Student Clinical Director, Teaching Assistant in Radiology. Dr. Morries is most proud of his research papers and awards, in America Academy of Pain Medicine, Sciatic and Suprascapular Nerve Blocks with Dr. Steve Gulevich, MD. He was asked to share two Poster presentations at the North American Laser Foundation in 2011on Low Back Pain, plus Polyneuropathy treatment with Laser (NIR) therapy. His Podium Presentation and publication on Hip dysplasia, in American Board of Chiropractic Sports Physicians®. Additionally, he has given presentations abroad at State of Chiropractic Research, Foundation of Chiropractic Education and Research, in Bournemouth England and Vancouver, BC, Canada. Dr. Theodore Henderson has extensive training and experience to the practice of Psychiatry. He trained in Psychiatry at the prestigious Barnes/Jewish Hospitals at Washington University in St. Louis. Dr. Henderson completed a fellowship in Child & Adolescent Psychiatry at the University of Colorado. He also has training in Radiology, Nuclear Medicine, and the genetics of psychiatry. He established his private practice in Centennial Colorado in July of 2000. Dr. Henderson brings a unique blend of expertise in psychopharmacology, neurobiology, and an understanding of human nature to the practice of psychiatry. Dr. Henderson attended medical school at Saint Louis University School of Medicine. While in medical school, he began studying heart pathology under Dr. Vernon Fischer. He earned an American Heart Association Medical Student Research Fellowship. With this fellowship, he spent one year at the University of Washington studying the pathology of atherosclerosis. In 1991, Dr. Henderson founded the Child Abuse Prevention Task Force at Saint Louis University. This program taught children, parents, and teachers about child sexual abuse and how to prevent it. Each year, this program reached over 8,000 children throughout the metro St. Louis area, primarily in the poor inner-city schools. The program was awarded numerous awards, including a Saint Louis University Community Service Award, Commendations from the school districts, and an award from the American Medical Student Association. Dr. Henderson was nominated for a Student Life Leadership Award and earned a Departmental Award from the Department of Community and Family Medicine. He also received a Weis Humanitarian Award recognizing outstanding humanitarian care as a medical student. Dr. Henderson wrote a training manual on this program that was implemented at other medical schools and he cowrote a book chapter in the book, A Parent’s & Teacher’s Handbook on Identifying and Preventing Child Abuse (1998). During graduate school and medical school, Dr. Henderson published numerous research studies. He published 9 articles and 27 abstracts about his research in brain development. He also published a book chapter on brain development in collaboration with his research professor, Dr. Mark Jacquin. His research focused on the role of neural growth factors and impulse activity on the development of brain organization. He collaborated with leading researchers, including Drs. Thomas Woolsey, Eugene Johnson, and Thomas Rhoades. While a medical student, Dr. Henderson wrote two research grants (as part of program project grants). Both were funded. He continued conducting research at Saint Louis University and Washington University throughout his residencies. Dr. Henderson trained for one year in Radiology, focusing on neuroimaging and pediatrics. With this strong base, he then undertook a residency in Psychiatry at Washington University’s program at Barnes/Jewish Hospitals in St. Louis. His residency included extended training in general pediatrics at St. Louis Children’s Hospital. In 1997, He was awarded the National Institute of Mental Health Outstanding Resident Award for his ongoing work in child abuse prevention and his neurobiological research while a resident. Dr. Henderson completed a residency in Adult (or General) Psychiatry and then undertook a fellowship in Child Psychiatry at the University of Colorado. This included additional specialization in Autism and Autism Spectrum Disorders. He compl
Effect of autologous mesenchymal stem cells induced by low level laser therapy on cardiogenesis in the infarcted area following myocardial infarction in rats
Hana Tuby1, Tali Yaakobi1, Lidya Maltz1, Yaakov Delarea2, Orit Sagi-Assif2, Uri Oron1* - (Publication) 4467This study showed rats that were give a heart attack and then treated with the laser on their shins saw a 55% reduction in infarction size in the heart showing that the stem cells released from the bone migrated to the heart.
1Department of Zoology, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv, Israel
2Department of Cell Biology and Immunology, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv, Israel
Email: *oronu@post.tau.ac.il
Received 27 May 2013; revised 29 June 2013; accepted 16 July 2013
ABSTRACT
In this study, we investigated the hypothesis that photo- biostimulation by low-energy laser therapy (LLLT) applied to the bone marrow (BM) of myocardial in- farcted rats may attenuate the scarring processes that follow myocardial infarction (MI). Wistar rats under- went experimental MI. LLLT (Ga-Al-As diode laser) was applied to the BM of the exposed tibia at differ- ent time intervals post-MI (4 hrs, 48 hrs and 5 days). Sham-operated infarcted rats served as control. In- farct size was significantly reduced (55%) in the la- ser-treated rats as compared to the control non-treat- ed rats, at 2 weeks post-MI. A significant 3-fold in- crease was observed in the density of desmin immu- nopositive stained cells 14 days post-MI in the infarc- ted area of the laser-treated rats as compared to the non-laser-treated controls. The electron microscopy from the control infarcted rat hearts revealed a typi- cal interphase area between the intact myocardium and the infarcted area, with conspicuous fibroblasts with collagen deposition dispersed among them. In rats that were laser treated (to BM), the interphase zone demonstrated cells with different intracellular struc- tures. There was also a significant increase in the per- centage of c-kit positive cells and macrophages in the circulating blood of the laser treated rats as compar- ed to control non treated ones. In the majority of the cells clusters of myofibrils anchored to well-developed Z-lines and structures resembling the morphological characteristics of mature intact cardiomyocytes were evident. In conclusion, LLLT to the BM of rats post- MI induces cardiogenesis mainly at the borders of the infarcted area in the heart.
Keywords: Low-Level Laser Therapy; Myocardial Infarction; Macrophage; Desmin; Ultrastructure; c-Kit Positive Cells
1. INTRODUCTION
Regenerative capacity and mitotic activity in the heart are confined mainly to the lower vertebrates [1]. Amputation of ~20% of the zebrafish’s ventricular myocardium re- sulted in full regeneration without scarring [2]. In am- phibians, heart injury was associated with increased cell proliferation of myocytes and enhanced regeneration [3]. The adult mammalian heart was traditionally considered to be a post-mitotic organ with terminally differentiated cardiac myocytes. However, this dogma has recently been challenged by several studies and reviews [4-8]. These studies have suggested that cardiac myocytes are replaced throughout the lifespan even in the human heart, and that myocytes can regenerate from resident cardiac progenitor cells (CPC) as well as from bone marrow (BM). Studies in human infarcted hearts have shown evidence of cytoki- nesis of cells in the heart and evidence of cardiac stem cells that are activated in response to ischemic injury. This growth response is attenuated in chronic heart fail- ure [9]. Some studies have reported that cardiac myocyt- es can be derived from BM; specifically, side population precursor cells following induction of myocardial infarc- tion (MI) by left anterior descending artery (LAD) liga- tion [10-12]. Contradicting these findings, other laborato- ries using genetic markers have reported that lineage ne- gative, c-kit+ BM cells did not differentiate into cardio- myocytes [13]. It was also suggested that BM-derived stem cells may stimulate the small population of stem cells in the ischemic heart to proliferate and differentiate to enhance cardiac repair post-MI [14]. In a recent study transient regenerative potential in the mouse heart was demonstrated during the neonatal period [15].
Low-level laser therapy (LLLT) has been found to modulate various biological processes [16,17], such as increasing mitochondrial respiration and ATP synthesis [18], facilitating wound healing and promoting the proc- ess of skeletal muscle regeneration and angiogenesis [19- 21]. In an experimental model of the infarcted heart in rats and dogs, it was demonstrated that LLLT application directly to the infarcted area in the heart at optimal power parameters significantly reduced scar tissue formation [22-24]. This phenomenon was partially attributed to a significant elevation in ATP content, heat shock proteins, vascular endothelial growth factor (VEGF), inducible ni- tric oxide (NO) synthase, and angiogenesis in the ischemic zone of the laser-irradiated rats, as compared to non- irradiated rats [25].
The effect of photobiostimulation on stem cells or pro- genitor cells has not been extensively studied. LLLT ap- plication to normal human neural progenitor cells signi- ficantly increases ATP production in these cells [26]. LLLT delivery to MSCs and cardiac stem cells in vitro caused a significant enhancement in their proliferation rate [27,28]. LLLT has also been shown to increase the proliferation rate of adipose-derived stem cells in vitro [29]. Recently, we demonstrated that LLLT application to autologous BM could induce mesenchymal stem cells (MSCs) in the BM to proliferate and cause their recruit- ment and specific homing in on the infarcted rat heart and not on other organs [30,31]. The laser treatment to the BM also caused a marked and statistically significant reduction of 79% in the scarring and ventricular dilata- tion followed MI as compared to infarcted non-laser- treated rats. The aim of the present study was to investi- gate the possibility that induction of stem cells in the BM of rats by LLLT could also affect cardiogenesis in the in- farcted rat heart.
2. MATERIALS AND METHODS
2.1. Experimental Procedures
A total of 21 Wistar male rats, weighing 200 - 250 gr, that underwent ligation of the LAD artery to induce MI, were used as described by us previously [23]. All the ex- perimental procedures were approved by the animal care committee of Tel-Aviv University. Briefly, rats were anes- thetized with Avertin (1 ml/100 g body weight I.P.) and the lungs were ventilated. Thoractomy was performed by invasion of the intercostals muscles between the 5th and 6th rib to expose the heart. The LAD artery was occluded 2 mm from the origin with 5-0 polypropylene thread (Ethicon Inc., Cincinnati, OH). Following LAD artery occlusion the chest muscles and skin were sutured and the rats were ventilated until they woke up. The infarcted rats were divided randomly into two groups. In one group LLLT was applied directly to the BM 4 hrs, 48 hrs and 5 days post-MI (see below). The second group was non-laser-treated (the rat’s bone was exposed for the same duration as the laser-treated group but the laser was not turned on). Food and water were supplied ad libitum. Rats were sacrificed 14 days post-MI.
2.2. Laser Application
After induction of MI rats were randomly assigned to a laser-treated or control non-laser-treated group. A diode (Ga-Al-As) laser, wavelength 804 nm with a tunable po- wer output of maximum of 400 mW (Lasotronic Inc., Zug, Switzerland) for application to the BM was used. The laser device was equipped with a metal-backed glass fiber optic (1.5 mm diameter). An infrared viewer (Laso- tronic Inc. Zug, Switzerland) and infrared-sensitive de-tecting card (Newport, Inc., Irvine, CA) were used to de- termine the infrared irradiation area. Laser application was done by a 10 mm longitudinal cut in the skin above the medial aspect, and further delicate cleaning of the bone surface was carried out. The tip of the fiber optic (1.5 mm diameter) was placed perpendicularly to the center of the exposed medial aspect of the tibia and power den- sity of 10 mW/cm2 was applied to the BM. The laser was applied for a duration of 100 sec (energy density 1.0 J/cm2). Left or right exposed tibias were chosen at random for LLLT application. In sham-operated infarcted rats that served as control the tibias were exposed and the fi- ber optic was placed as described above but the laser beam was not turned on.
2.3. Histology and Electron Microscopy
A defined cross-section sample (2 mm thick) from the central part of the infarcted area was taken from all hearts for histology. Eight micron paraffin sections were pre- pared from the tissue samples of each heart. Infarct size was determined using Masson’s trichrome staining as described by us previously [23]. Three observers, blinded to control or laser-treated rats, analyzed infarct size. Six microscopic slides from the infarcted area of each heart were chosen at random for determination of infarct size. Infarct size was expressed as the percentage of the total infarcted area relative to the total area of the left ventri- cle (LV) in each section, using image analysis software Sigma Scan Pro (Sigma, St. Louis, MO).
For electron microscopy three tissue samples from each of the control and laser-irradiated rat hearts were taken from the interphase zone between the infarcted and non-infarcted tissue by macroscopic examination. Fixa- tion was performed in 3.5% glutaraldehyde in 0.1 M ca- codylate buffer for 24 hrs followed by embedment in Epon-812. Semi-thin sections (1 micron) were prepared in order to localize the interphase zone. Thin sections were then prepared and stained with uranyl acetate and lead citrate followed by examination with a Jeol electron microscope.
2.4. Immunohistochemistry
The total number of cells immunostained for desmin (bone marrow cells or newly formed) in the infarcted area were determined using a desmin kit (Zytomed Laboratory, Ber- lin, Germany). The procedure was performed at room temperature with anti-mouse (dilution 1:25 - 1:50) primary antibody for 60 min. Following washing, slides were in- cubated with HRP secondary antibody for mouse for 30 min followed by DAB Chromogen system (Covance Inc., Dedham). Slides were rinsed again in wash buffer, stain- ed in Hematoxylin for nuclei detection, mounted and viewed using a Zeiss microscope equipped with a camera and video screen. The total number of desmin immuno- stained cells within the infarcted area was counted and their density expressed as the percentage of the total area of the infarct using SigmaPro software.
2.5. Flow Cytometry Analysis
Blood samples were taken 2 and 7 days post-IR injury for fluorescence-activated cell sorting (FACS) analysis. 100 μl of blood were mixed with different antibodies: anti-mouse CD117 (c-kit) PE (eBioscience San Diego, USA) and rat IgG2b isotype control PE (eBioscience San Diego, USA) and anti-rat macrophage marker PE (eBio- science San Diego, USA) and mouse IgG2a K isotype control PE (eBioscience San Diego, USA), were used for the FACS analysis according to the manufacturer’s guide- lines. Forty five min post incubation of the whole fresh blood with the relevant antibodies, 2 ml of Fix/Lyse so- lution (eBioscience, San Diego, USA) was added. After mixture the suspended cells were left for 60 min in the dark at room temperature. Centrifugation was performed for 10 min, supernatant was removed and washing of the pellet was performed with 2 ml of Flow Cytometry Stain- ing Buffer Solution (eBioscience, San Diego, California, USA). After another centrifugation for 10 minutes the supernatant was decanted. The pellet containing mono- nucleated cells was resuspended in 200 μl of flow stain buffer for FACS analysis.
2.6. Statistical Analysis
The SigmaStat 2.0 (Sigma, St. Luis, USA) software was used for statistical analysis. Tests were performed first for normality distribution, followed by parametric (stu- dent’s t-test) test.
3. RESULTS
Application of LLLT to the infarcted heart caused a sig- nificant (p = 0.049) reduction of 55% in infarct size as compared to control. The present of macrophages and c- kit positive cells in the blood was determined by FACS analysis (Figure 1). It was found that at 5 days post MI there was a statistical significant 2-fold higher concentra- tion of macrophages and significant 1.4-fold higher c-kit positive cells (mesenchymal cells) in the laser treated rats as compared to the infarcted non laser treated rats. Des- min immunostaining of histological sections of the in- farcted zone from laser-treated rats demonstrated a higher density of positively stained cells than in the non laser-treated ones (Figures 2-4). In the interphase zone, cells extending from the myocardium towards the in 
Figure 1. Percent (out of total mononucleated cells) of macro- phages and c-kit positive cells in blood of control and laser treated rats (to the bone marrow) 5 days post MI as revealed by FACS analysis. The results are mean ± S.E.M of 15 rats at each group. Statistical significance *p < 0.05; **p < 0.01.

Figure 2. Representative desmin immunostained light micro- graphs of the infarcted zone of non-laser-treated rats (a, c) and laser-treated rats (to the bone marrow at 4 and 48 hrs and 5 days) (b, d) taken 2 weeks post-MI. Note that the zone in the control non-laser-treated rats contains mainly collageneous mate- rial with a few desmin immunopositive cells in the infarcted area (a, c); while in the laser-treated rats the zone displays posi- tive desmin staining in extended outgrowths (arrow) from the myocardium (MC) in (b), and in the cytoplasm of many cells in the infarcted area in (d). IF, Infarcted area. Bar = 50 μm.
farcted area showed higher immunostaining for desmin in the laser-treated rat hearts as compared to the control non-treated ones (Figure 2). The cell density of desmin immune-positive cells was also determined quantitatively in histological sections of both the infarcted laser-treated rats and infarcted non-laser-treated rats. The cell density was significantly (p < 0.01) 3-fold higher in the infarcted area of the laser-treated rats as compared to the non-la- ser-treated controls (Figure 4).
The electron micrographs of all samples taken from the control non-laser-treated infarcted rat hearts revealed a typical interphase area between intact and infarcted heart (Figure 5(a)). Adjacent to the non-ischemic intact myocardium there were conspicuous fibroblasts with col- lagen deposition dispersed among them (Figure 5(a)). In all samples taken from the laser-irradiated hearts the in- terphase zone between intact and infarcted area demon- strated different characteristics to those of the non-laser- treated infarcted rat hearts. Cells with newly-formed or- ganized contractile myofilaments dispersed in the cyto- plasm were detected in groups of several cells (Figure 5(b)). In these cells numerous mitochondria, clusters of ribosomes, and conspicuous clusters of contractile pro- teins were evident in the cytoplasm (Figures 6-8). Some cells contained dispersed contractile myofilaments in the cytoplasm that were still in an early stage of organization (Figure 6). The organization of newly-formed contractile myofilaments in the cytoplasm was observed in various

Figure 3. Representative desmin immunostained light micro- graphs of the interphase of the infarcted zone of laser-treated rats. Note that desmin positively stained cross-sections of myo- fibers (arrows) intermingled in the infarcted zone in (a). In (b) immunopositively stained cross-sections of myofibers (arrow) are visible in the infarcted area (IF). In (c) newly-formed car- diomyocytes (NC) are seen, with the desmin immunostaining mainly confined to the Z-line. Bar = 50 μm.

Figure 4. Density of desmin positively stained area (relative to total area) in the infarcted areas of control (non-laser-treated) and laser-treated (to the bone marrow) rats at 14 days post-MI. Results are mean+ S.E.M from 6 - 8 rats in each group. **p < 0.01.

Figure 5. Electron micrographs of typical interphase zone be- tween myocardium and infarcted area of control non-laser- treated (a) and laser-treated (b) to bone marrow rats. Note intact myocardium (MY) and adjacent fibroblast (FB) in the infarcted area surrounded by collagen (CL) deposition in (a). In (b) sev- eral newly-formed cardiomyocytes (marked with asterix) with conspicuous well-organized myofilaments (MF) in their cyto- plasm are evident adjacent to blood capillaries (CA). EN, En- dothelial cell.
degrees of maturation in those cells. In some cells the myofilaments were dispersed in the cytoplasm and in others they were organized in clusters anchored to well- developed Z-lines (Figure 7(a)). In certain cells the myo- filaments were organized parallel to the longitudinal di- rection of the cells, resembling the morphological char- acteristics of mature intact cardiomyocytes (Figure 7(b)). Some of the cells were also seen in a process of forma- tion of typical intercalated disc between them (Figure 9).
4. DISCUSSION AND CONCLUSION
The most significant outcome of this study was the ap- pearance of newly-formed cardiomyocytes following laser treatment to the BM, as indicated by light and electron microscopy. There was a 3-fold increase in the density of

Figure 6. Electron micrographs of most probably newly-formed cardiomyocytes at an early stage of organization of contractile myofilaments. Note myofilaments (MF) in the cytoplasm. M, Mitochondrion. Bar = 1 μm.

Figure 7. Electron micrographs of most probably newly-formed cardiomyocytes with early (a) and late (b) stages of the organi- zation of the contractile myofilaments in the cytoplasm. Note contractile myofilaments that are dispersed (DMF) in the cyto- plasm with a few organized in clusters anchored to Z-lines (Z) in (a). In (b) myofilaments (MF) are organized in parallel to the longitudinal axis of the cardiomyocyte, resembling their orga- nization in mature cardiomyocyte. N, Nucleus. Bar = 1 μm.
desmin immunostained cells in the infarcted rat hearts that had been laser treated. Desmin is a protein found in the cytoplasm of developing myocytes and cardiomyo- cytes [32]. The significantly higher occurrence of des- min-positive cells in the infarcted area of the laser- treated hearts may indicate the synthesis of new contrac- tile proteins in the developing new cardiomyocytes, re- sembling the process that takes place during embryonic development. The ultrastructural features of the cells in the interphase between the intact myocardium and the

Figure 8. Electron micrographs of typical interphase zone be- tween myocardium and infarcted area of laser-treated infarcted rat heart. Note numerous mitochondria (M) in the cytoplasm of the cardiomyocytes in (a) and (b). Also note organized contrac- tile myofilament with well-developed Z-lines (Z), some dis- persed myofilaments and clusters of ribosomes (R). Bar = 1 μm.

Figure 9. Electron micrographs of typical intercalated disk formation in the interphase region of the infarcted heart of la- ser-treated rats. Formation of intercalated disks (ID) between cells (marked with asterix) is evident. Note that the most proba- bly newly-formed cardiomyocytes contain clusters of myofila- ments (MF) in the cytoplasm that are conspicuous in their obli- que or cross-sections (arrows). Bar = 1 μm.
infarcted myocardium of the laser-treated rats, as shown in this study, clearly resemble the characteristics of car- diomyocytes during embryonic development of the heart [33]. Furthermore, the clusters of ribosomes and the nu- merous clusters of mitochondria in the cytoplasm of these cells may characterize cells that are active in the synthe- sis of proteins. It was previously demonstrated that direct LLLT to the infarcted hearts of rats, dogs and pigs caus- ed a significant reduction of scarring post-MI [23,24]. It was suggested that part of this reduction could be ex- plained by the regenerative response that takes place in the interphase zone [24].
The results of the present study indicate that the LLLT
applied to autologous BM attenuates the concentration of macrophages and MSC in the circulating blood. We have previously shown that LLLT application to the BM of infarcted rats caused a 2 fold enhancement in the rate of proliferation of MSC in the BM [30]. Those cells that most probably leave the BM to the circulating blood in- deed show a significant elevation of their concentration (as reveled by the FACS analysis in the present paper) at 5 days post MI. Consequently these cells probably home in on the infarcted heart, and even migrate specifically to the infarcted area [30]. These cells may induce cardiac stem cells to differentiate to newly-formed cardiomyo- cytes, as suggested previously by Hatzistergos et al. [14]. Indeed, it was found that endogenous c-kit+ cardiac stem cells were increased by 20-fold in the rat infarcted heart compared to control, following transcardial injection of BM-derived MSCs [14]. Such induction may be enabled due to paracrine secretion of various growth factors by the laser-stimulated MSC that originated from the BM. The possibility that paracrine secretion occurs in im- planted stem cells during cell therapy to the heart post- MI has been suggested previously [34]. Another mecha- nism that may take place after homing of stem cells to the infarcted heart of the laser-stimulated rats is that these cells continue to proliferate in the appropriate mi-lieu of the interphase zone in the infarcted heart and then differentiate to cardiomyocytes [30].
Another possible mechanism that maybe associates with the reduction of infarct size is the significant increase in the concentration of macrophages in the circulation fol- lowing LLLT to the BM as revealed from the FACS analysis in the present study. These findings corroborate with studies indicating that macrophages activity in the infarcted area at early stages post MI cause reduction of scarring post MI [35,36]. Thus, it could be postulated that more macrophages that will eventually home in the infarcted area from the circulating blood in the laser treated rats will also contribute to the reduction of scar- ring.
Although the findings of the present study do not in- dicate the extent of regenerative capacity of the rat in- farcted heart post-laser-irradiation, they do reveal a shift from practically no cardiomyocytes in the tissue samples taken from the non-laser-treated hearts, to the presence of newly-formed cardiomyocytes in all the electron mi- croscope sections taken from the hearts of rats that are laser-treated to the BM.
In conclusion, to the best of our knowledge, this is the first study to demonstrate the appearance of newly-form- ed cardiomyocytes in the infarcted area following LLLT to autologous BM in the infarcted rat heart. The mecha- nisms associated with this phenomenon remain to be elu- cidated in further studies.
5. ACKNOWLEDGEMENTS
This study was partially supported by the Elizabeth and Nicholas Shle- zak Super-center for Cardiac Research and Medical Engineering. The authors wish to acknowledge N. Paz for editing the manuscript and V. Wexler for helping with preparation of the figures.
REFERENCES
[1]
Rumyantsev, P.P. (1977) Interrelations of the prolifera- tion and differentiation processes during cardiac myoge- nesis and regeneration. International Review of Cytology, 51, 186-273. doi:10.1016/S0074-7696(08)60228-4
[2]
Poss, K.D., Wilson, L.G. and Keating, M.T. (2002) Heart regeneration in zebrafish. Science, 298, 2188-2190. doi:10.1126/science.1077857
[3]
Rumyantsev, P.P. (1973) Post-injury DNA synthesis, mi- tosis and ultrastructural reorganization of adult frog car- diac myocytes. An electron microscopic-autoradiographic study. Z Zellforsch Mikrosk Anat, 139, 431-50. doi:10.1007/BF00306596
[4]
Barnett, P. and van den Hoff, M.J.B. (2011) Cardiac re- generation: Different cells same goal. Medical & Biologi- cal Engineering & Computing, 49, 723-732. doi:10.1007/s11517-011-0776-5
[5]
Bollini, S., Smart. N. and Riley, P.R. (2011) Resident car- diac progenitor cells: At the heart of regeneration. Jour- nal of Molecular and Cellular Cardiology, 50, 296-303. doi:10.1016/j.yjmcc.2010.07.006
[6]
Choi, W.Y. and Poss, K.D. (2012) Cardiac regeneration. Current Topics in Developmental Biology, 100, 319-343. doi:10.1016/B978-0-12-387786-4.00010-5
[7]
Laflamme, M.A. and Murry, C.E. (2011) Heart regenera- tion. Nature, 473, 326-335. doi:10.1038/nature10147
[8]
Steinhauser, M.L. and Lee, R.T. (2011) Regeneration of the heart. EMBO Molecular Medicine, 3, 701-712. doi:10.1002/emmm.201100175
[9]
Urbanek, K., Torella, D., Sheikh, F., De Angelis, A., Nur- zynska, D., Silvestri, F., Beltrami, C.A., Bussani, R., Bel- trami, A.P., Quaini, F., Bolli, R., Leri, A., Kajstura. J. and Anversa, P. (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proceedings of the National Academy of Sciences of the USA, 102, 8692-8697. doi:10.1073/pnas.0500169102
[10]
Bittner, R.E., Schofer, C., Weipoltshammer, K., Ivanova, S., Streubel, B., Hauser, E., Freilinger, M., Höger, H., Elbe- Bürger, A. and Wachtler, F. (1999) Recruitment of bone- marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anatomy and Embryology, 199, 391-396. doi:10.1007/s004290050237
[11]
Jackson, K.A., Majka, S.M., Wand, H., Pocius, J., Hart- ley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation, 107, 1395-1402. doi:10.1172/JCI12150
[12]
Pfister, O., Mouquet, F., Jain, M., Summer, R., Helmes, M., Fine, A., Colucci, W.S. and Liao, R. (2005) CD3- but not CD31+ cardiac side population cells exhibit functio- nal cardiomyogenic differentiation. Circulation Research, 97, 52-61. doi:10.1161/01.RES.0000173297.53793.fa
[13]
Balsam, L.B., Wagers, A.J., Christensen, J.L., Kofidis, T., Weissman, I.L. and Robbins, R.C. (2004) Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature, 428, 668-673. doi:10.1038/nature02460
[14]
Hatzistergos, K.E., Quevedo, H., Oskouei. B.N., Hu, Q., Feigenbaum, G.S., Margitich, I.S., Mazhari, R., Boyle, A.J., Zambrano, J.P., Rodriguez, J.E., Dulce, R., Pattany, P.M., Valdes, D., Revilla, C., Heldman, A.W., McNiece, I. and Hare, J.M. (2010) Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differ- entiation. Circulation Research, 107, 913-922. doi:10.1161/CIRCRESAHA.110.222703
[15]
Porrello, E.R., Mahmoud, A.I., Simpson, E., Hill, J.A., Richardson, J.A., Olson, E.N. and Sadek, H.A. (2011) Transient regenerative potential of the neonatal mouse heart. Science, 331, 1078-1080. doi:10.1126/science.1200708
[16]
Conlan, M.J., Rapley, J.W. and Cobb, C.M. (1996) Bio- stimulation of wound healing by low energy laser irradia- tion. Journal of Clinical Periodontology, 23, 492-496. doi:10.1111/j.1600-051X.1996.tb00580.x
[17]
Karu, T. (2007) Ten lectures on basic science of laser photherapy. Prima Books, Gragesberg.
[18]
Karu, T. (2010) Mitochondrial mechanisms of photobio- modulation in context of new data about multiple roles of ATP. Photomedicine and Laser Surgery, 28, 159-160. doi:10.1089/pho.2010.2789
[19]
Bibikova, A. and Oron, U. (1993) Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius mu- scle by low energy laser irradiation. Anatomical Record, 235, 374-380. doi:10.1002/ar.1092350306
[20]
Bibikova, A., Belkin, A. and Oron, U. (1994) Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low energy laser irradiation. Anatomy and Embryology, 190, 597-602. doi:10.1007/BF00190110
[21]
Oron, U. (2006) Photoengineering of tissue repair in ske- letal and cardiac muscles. Photomedicine and Laser Sur- gery, 24, 111-120. doi:10.1089/pho.2006.24.111
[22]
Yaakobi, T., Shoshani, Y., Levkovitz, S., Rubin, O., Ben- Haim, S.A. and Oron, U. (2001) Long term effect of low energy laser irradiation on infarction and reperfusion in- jury in the rat heart. Journal of Applied Physiology, 90, 2411-2441.
[23]
Oron, U., Yaakobi, T., Oron, A., Hayam, G., Gepstein, L., Wolf, T., Rubin, O. and Ben Haim, S.A. (2001a) Attenu- ation of the formation of scar tissue in rats and dogs post myocardial infarction by low energy laser irradiation. La- sers in Surgery and Medicine, 28, 204-211. doi:10.1002/lsm.1039
[24]
Oron, U., Yaakobi, T., Oron, A., Mordechovitz, D., Shof- ti, R., Hayam, G., Dror, U., Gepstein, L., Wolf, T., Hau- denschild, C. and Ben Haim, S.A. (2001b) Low energy la- ser irradiation reduces formation of scar tissue following myocardial infarction in dogs. Circulation, 103, 296-301.doi:10.1161/01.CIR.103.2.296
[25]
Tuby, H., Maltz, L. and Oron, U. (2006) Modulations of VEGF and iNOS in the rat heart by low energy laser irra- diation are associated with cardioprotection and enhanced angiogenesis. Lasers in Surgery and Medicine, 38, 682- 688. doi:10.1002/lsm.20377
[26]
Oron, U., Ilic, S., De Taboada, L. and Streeter, J. (2007) Ga-As (808 nm) laser irradiation enhance ATP produc- tion in human neuronal cells in culture. Photomedicine and Laser Surgery, 25, 180-182. doi:10.1089/pho.2007.2064
[27]
Tuby, H., Maltz, L. and Oron, U. (2007) Low-level laser irradiation (LLLI) promotes proliferation of mesenchy- mal and cardiac stem cells in culture. Lasers in Surgery and Medicine, 39, 373-378. doi:10.1002/lsm.20492
[28]
Li, W.T., Leu, Y.C. and Wu, J.L. (2010) Red-light light- emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchy- mal stem cells. Photomedicine and Laser Surgery, 28, S-157-S-165. doi:10.1089/pho.2009.2540
[29]
Mvula, B., Moore, T.J. and Abrahamse, H. (2010) Effect of low-level laser irradiation and epidermal growth factor on adult human adipose-derived stem cells. Lasers in Medical Science, 25, 33-39. doi:10.1007/s10103-008-0636-1
[30]
Tuby, H., Maltz, L. and Oron, U. (2011) Induction of au- tologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers in Surgery and Medicine, 43, 401-409. doi:10.1002/lsm.21063
[31]
Oron, U. (2011) Light therapy and stem cells: A thera- peutic intervention of the future. Journal of Interventio- nal Cardiology, 3, 627-629.
[32]
Toma, C., Pittenger, M.F., Cahill, K.S., Byrne, B.J. and Kessler, P.D. (2002) Human mesenchymal stem cells dif- ferentiate to a cardiomyocyte phenotype in the adult mu- rine heart. Circulation, 105, 93-98. doi:10.1161/hc0102.101442
[33]
Oron, U. and Mandelberg, M. (1985) Focal regeneration in the rat myocardium following cold injury. Cell Tissue Research, 241, 459-463. doi:10.1007/BF00217194
[34]
Mummery, C.L., Davis, R.P. and Krieger, J.E. (2010) Challenges in using stem cells for cardiac repair. Science Translational Medicine, 14, 1-5.
[35]
van Amerongen, M.J., Harmsen, M.C., van Rooijen, N., Petersen, A.H. and van Luyn, M.J. (2007) Macrophage dep- letion impairs wound healing and increases left ventricu- lar remodeling after myocardial injury in mice. American Journal of Pathology, 170, 1093-1103. doi:10.2353/ajpath.2007.060547
[36]
Okazaki, T., Ebihara, S., Asada, M., Yamanda, S., Saijo, Y., Shiraishi, Y., Ebihara, T., Niu, K., Mei, H., Arai, H. and Yambe, T. (2007) Macrophage colony-stimulating factor improves cardiac function after ischemic injury by induc- ing vascular endothelial growth factor production and sur- vival of cardiomyocytes. American Journal of Pathology, 171, 1093-1103. doi:10.2353/ajpath.2007.061191
Original Source: http://www.scirp.org/journal/jbise
Influence of Low Level Laser Radiation on Migration of Stem Cells
Levon Gasparyan, Grigory Brill, Anu Makela - (Publication) 4468This study showed a 26% increase in stem cell when they uses red and IR lasers continuous wave.
Abstract: The long term effects of low level laser therapy can involve treatment mechanisms connected with activation of stem cells.
In the current study migration of stem cells was tested under the influence of laser light alone as well as in case of combined influence of light and stromal cell-derived factor-1α (SDF-1α). This cytokine plays a role in lymphocyte trafficking, hematopoietic progenitor cell and stem cell homing.
To investigate the light influence on stem cells, we analyzed factor-dependent cell-Patersen (FDCP)-mix multipotent progenitor cells.
Migration of the stem cell line was tested using Transwell system (Corning, NY) under influence of red diode laser (λ=659.6 nm, 19.5 mW) or infrared diode laser (λ=958 nm, 36 mW) during 15 min at continuous wave, as well as in case of applying 150 ng/ml SDF-1α.
Group 1 cells were a group of control, group 2 cells received only red light irradiation, while group 3 cells had IR light irradiation. Group 4 cells were treated with 150 ng/ml SDF-1α. Group 5 cells were irradiated with red laser light in addition to 150 ng/ml SDF-1α, and group 6 cells by IR light and 150 ng/ml SDF-1α.
The count of migrated cells was 1496,5±409 (100%) in case of control. Red and IR laser light increased migration activity of stem cells up to 1892±283 (126%) and 2255,5±510 (151%) accordingly. Influence of SDF-1α was more significant, than effects of light irradiation alone 3365,5±489 (225%). Combined effects of light irradiation and SDF-1α were significantly stronger 5813±1199 (388%) for SDF-1α and red laser light, and 6391,5±540 (427%) for SDF-1α and IR laser light irradiation.
Preliminary study results showed that laser light irradiation can activate stem cell migration in vitro. The results are more reliable in the case of combined application of light and SDF-1α. These results are giving ground to consider that stem cell reactions to light irradiation can be one of the factors of light therapy.
Key words: low level laser irradiation, low level laser therapy, stem cells, SDF-1, stromal cell-derived factor-1
INTRODUCTION
More than 30 years ago first reports about biological effects of low doses of laser light were presented. Currently low level laser therapy (LLLT) is successfully applied in the treatment of numerous diseases and pathological conditions. LLLT exhibits positive effects for the treatment of disorders, having in common failure of blood supply with development of acute or chronic tissue hypoxia, different level of destruction of tissues, following decreased regenerative abilities of tissues and organs, defects in immune system, and altered cell metabolism. At the same time some important mechanisms of influence of laser light on the body are still far to be fully understood [1 - 8].
Recent studies discovered important role of bone marrow hematopoetic stem cell (HSCs) for naturally occurred recovery and regeneration processes, following tissue hypoxia and injury. The three clinically important steps in this natural process are mobilization of stem cells from the bone marrow, homing of these cells to the site of injury, and differentiation of the stem cell into a functional cell of the injured tissue [9]. Different methods of stem cell therapy, the treatment method, based on mobilization and transplantation of stem cells, proves to be effective method of therapy for different disorders.
We proposed a hypothesis that wide range of positive effects following laser therapy can be connected to increased activity of stem cells in damaged tissues. To test that, we examined in vitro the influence of laser light on migration of stem cells in absence and in presence of stromal cell-derived factor-1 (SDF-1), a potent chemoattractor for lymphocytes, monocytes, HSCs, which plays a critical role in the stem cell migration towards areas of tissue injury and hypoxia.
MATERIALS AND METHODS
To investigate the light influence on stem cells, we analyzed factor-dependent cell-Patersen (FDCP)-mix multipotent progenitor cells. The FDCP-mix stem cell line was maintained in ISCOVE’S medium supplemented with 20% horse serum and penicillin/streptomycin in the presence of 20 ng/ml IL-3. The cells were supplied with fresh medium each 5 days. Migration of the stem cell line was tested using Transwell system (Corning, NY). The cells were washed with PBS once and re-suspended in the medium containing 0.1% BSA (2x106/ml). Then, 600 μl of the mixture was irradiated by red diode laser (λ=659.6 nm, 19.5 mW) or infrared diode laser (λ=958 nm, 36 mW) during 15 min at continuous wave. Next, 100 μl of the mixture (2x105 cells) was seeded into upper chambers of the Transwell system, and the filters were placed into the wells containing 600? μl of the medium with or without 150 ng/ml SDF-1α. The plate was incubated for 4 h (37°C, 5% CO2, humidified atmosphere), after which the cells were collected and counted by a FACS sorter (Beckton Dickinson) during 1 min. All samples were performed in duplicate.
Group 1 cells are control group, group 2 cells received only red light irradiation, while group 3 cells – only IR light irradiation. Group 4 cells were treated with 150 ng/ml SDF-1α. Group 5 cells were irradiated with red laser light in addition to 150 ng/ml SDF-1α, and group 6 cells – IR light and 150 ng/ml SDF-1α.
RESULTS
Small amount of stem cells can migrate without SDF-1α or laser light influence. The count of migrated cells in control group was 1496,5±409 (Fig). This amount was considered as 100%. Red and IR laser light at the above mentioned dosage and methods of irradiation increased migration activity of stem cells up to 1892±283 (126%) and 2255,5±510 (151%) accordingly. Influence of SDF-1α was more noticeable, than effects of red or IR laser light irradiation alone - 3365,5±489 (225%). It is important to stress attention on the finding, that rate of stem cell migration towards the filter and SDF-1α containing medium was much higher after laser irradiation of cells - 5813±1199 (388%) for red laser light, and 6391,5±540 (427%) for IR laser light irradiation.
DISCUSSION
The main scientific result of this study is the fact, that red and infrared laser light irradiation can activate migration of stem cells in vitro. Moreover, red and IR laser radiation can up-regulate the rate of stem cell migration towards higher SDF-1α gradient.
How to explain the direct effects of mobility of stem cells in vitro under red and IR laser light irradiation, and use this fact for better understanding the wide range of therapeutic effects of laser therapy?
Modern medical science has accepted that every pathologic condition or disease should be treated according to its clinical stage and symptoms, considering its pathogenesis and etiology. Similar treatment methods can be applied only for the treatment of different diseases, having common pathogenesis.
Not very many examples of successful application of the similar or close therapy method for the treatment of different pathologies are known in modern medicine. Steroid hormone therapy is one of such cases.
Another illustration of successful application of the similar treatment techniques for treatment of different disorders is stem cell therapy, a novel treatment method, which is still under development. Growing data suggests, that transplanted stem cell can successfully and for long period of time improve heart myocardial contractility and other heart functions after myocardial infarction, can support neoangiogenesis in areas of tissue infarction and damage, can replace several cell types in tissues, including β-cells in diabetes models, neurons, cardiomyocytes, hematopoetic cells of different lineages and so on, as well as be useful in the treatment of atherosclerosis [9].
The main principle of stem cell therapy is the idea of replacement of damaged and dead cells in injured tissues and organs with new healthy ones. It is known, that severe stress, tissue hypoxia and damage mobilizes some hematopoetic stem cells (HSCs) from bone marrow to peripheral bloodstream. After that HSCs can migrate towards hypoxic tissues and reach them. Finally they can start to proliferate to the cells types, typical for that damaged tissues. HSCs in the tissues are also able to produce several cytokines, chemokines, growthfactors, improve survival of damaged cells and limit apoptosis. As a result of some tissue regeneration, improvement in the function of a damaged organ can be achieved. Similar and even stronger regeneration and treatment effects can be displayed after transplantation of fetal or adult HSCs to recipient [10-12].
Low laser light irradiation is one other example of application of the same factor for the treatment of number of disorders, which, at first glance, have nothing or very little in common in their pathogenesis. Laser light can accelerate wound and burn healing, improve condition of patients after myocardial infarction and stroke, can support hematopoiesis of bone marrow after X-ray radiation or during cancer chemotherapy, can help for the treatment of diabetic angiopathy and neuropathy, as well as reduce atherosclerotic plaque formation. In cellular and tissue level LLLT exhibits positive effects for the treatment of disorders, having in common failure of blood supply with development of acute or chronic tissue hypoxia, different level of destruction of tissues, following with decreased regenerative abilities of cells, as well as altered cell metabolism [6, 7, 13, 14].
One can see that the therapeutic applications of LLLT and stem cell therapy are very close. So, earlier we proposed the hypotheses that one of the mechanisms of light therapy includes acceleration of tissue repair due to better mobilization of stem cells to the spot of injury after laser light irradiation [15]. That process should include several phases, including activation of stem cell migration towards area of tissue damage and hypoxia.
Stem cells are being investigated for their potential use in regenerative medicine. Stem cells share the following two defining characteristics: the capacity to differentiate into a spectrum of different cell types and the capacity to renew themselves [16]. The biological principle that underlies stem cell therapy is tissue-directed differentiation. For example, adult stem cells isolated from liver tissue and re-injected into liver become hepatocytes, whereas the same cells injected into myocardium become myocytes. [17] Stem cells have been engrafted into a broad spectrum of tissues, including regenerating bone, neural tissue, dystrophic skeletal muscle, and injured skeletal muscle. [18]. Myocardial regeneration is perhaps the most widely studied and debated example of stem cell plasticity. The most promising results have been obtained after transplantation and mobilization of bone marrow cells to the area of infarction.
The three clinically important steps in this natural process are mobilization of stem cells from the bone marrow, homing of these cells to the site of injury, and differentiation of the stem cell into a functional cell of the injured tissue [19].
Stem cell repair of cardiac and vascular tissue is a naturally occurring process after injury [20, 21] Circulating CD34+ mononuclear cell counts and plasma levels of endothelial growth factor are significantly increased in patients with acute myocardial infarction, peaking on day 7 after onset [22]. Due to limitations of the naturally occurring repair process after myocardium infarction and other injuries or pathologies several stem cell transplantation strategies were proposed and tested.
At present, however, enthusiasm for the therapeutic potential of strategies of stem cell transplantation is limited by certain practical considerations. For example, the number of stem cells, required for injection for the treatment of myocardial infarction, can be harvested approximately from 6 l of donor blood [23].
Other important limitation for autologous bone marrow stem/progenitor cell mobilization is a recent finding, that circulating endothelial progenitor cells in patients with coronary heart disease are impaired with respect to number and functional activity. Moreover, Heeschen et al [24] reported that regeneration and functional ability of bone marrow-derived mononuclear cells (BM-MNCs) in patients with chronic ischemic cardiomyopathy (ICMP) are also limited. In spite of the fact that, the number of BM-MNCs isolated from bone marrow aspirates of 18 patients with ICMP and 8 healthy subjects s did not differ, the colony-forming capacity of BM-MNCs from patients with ICMP was significantly lower compared with BM-MNCs from healthy controls. Likewise, the migratory response to SDF-1 and vascular endothelial growth factor (VEGF) was significantly reduced in BM-MNCs derived from patients with ICMP compared with BM-MNCs from healthy controls. The reduced neovascularization capacity in vivo of BM-MNCs derived from patients with ICMP closely correlated with the in vitro assessment of SDF-1-induced migration and colony-forming capacity.
The need for development of new methods for mobilization, as well as for homing of stem cells to the site of injury is therefore evident.
Several growth factors, chemokines and cytokines are involved in the regulation of stem cell mobilization, homing and differentiation. Stromal cell-derived factor-1 (SDF-1) is one of them. SDF-1 is a chemokine playing an important role in the trafficking of hematopoietic stem cells. SDF-1 is expressed on stromal cells of various tissues. CXCR4 is the only known receptor for SDF-1 [25]. SDF-1/CXCR4 interaction is reported to play an important physiological role during embryogenesis in hematopoiesis, vascular development, cardiogenesis, and cerebellar development [26-28].
Recently, several investigators have reported that CD34+ cells, classically considered to be hematopoietic stem cells, expressed CXCR4, and that SDF-1 could induce CD34+ cell migration in vitro [29]. Accordingly, SDF-1 is considered as one of the key regulators of hematopoietic stem cell trafficking between the peripheral circulation and bone marrow. SDF-1 has also been shown to effect CD34+ cell proliferation and mobilization and to induce angiogenesis in vivo [30 -32].
Hattori et al [31] reported that plasma elevation of SDF-1 induced mobilization of mature and immature hematopoietic progenitors and stem cells, including endothelial progenitor cells (EPCs). However, application of granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization is widely accepted nowadays.
Yamaguchi et al [23] studied the effects of SDF-1 on migration and accumulation of EPCs. SDF-1 induced EPCs migration in a dose dependent manner in vitro. The magnitude of migration was similar to that induced by VEGF. Authors also reported that locally (in hind-limb ischemic muscle of experimental animals) administered SDF-1 could augment the local accumulation of transplanted EPCs from peripheral blood, thereby resulting in enhanced neovascularization. As a result, cell transplantation not only improved neovascularization but also reduced adverse biological consequences such as limb necrosis and auto-amputation in the mouse ischemic hind-limb model. These studies also disclosed that systemic EPCs transplantation improved myocardial neovascularization and cardiac function corresponding to reduced left ventricular scarring. Authors concluded that, at least under the experimental conditions used in the study, the effect of SDF-1 on neovascularization appears to result primarily from its ability to enhance the recruitment and incorporation of transplanted EPCs.
Damas at al. [33] reported that SDF-1α, at least in high concentrations, may mediate anti-inflammatory and matrix-stabilizing effects in unstable angina. These effects may promote plaque stabilization, and therapeutic intervention that enhances SDF-1 α activity could potentially be beneficial in acute coronary syndromes. Authors demonstrated significantly altered SDF-1/CXCR4 expression in patients with angina, with particularly marked changes in those with unstable disease, with low SDF-1 levels in plasma and altered expression of its corresponding receptor on peripheral blood mononuclear cells (PBMC). In contrast to the raised plasma levels of inflammatory chemokines in patients with angina plasma levels of SDF-1 and the surface expression of its corresponding receptor (CXCR4) on PBMC appear to be down-regulated in these patients. Thus, although persistent inflammation may involve up-regulation of inflammatory chemokines, recent studies suggest that inflammatory cytokines (eg, TNF-α and IL-1) may decrease the expression of SDF-1 and CXCR4.
Future progress of stem therapy techniques probably will include development of incubation methods for enhancement stem cell mobility and homing ability, as well as for faster proliferation into desire tissue cells. Increasing migration abilities will help to achieve better and faster results.
The ability of laser light to activate migration and mobility of different cells is well known. It was noticed, that irradiation of sperm cells in vitro can increase their mobility and fertility [34]. Moreover, this effect is more pronounced in case of damaged cells with low mobility rate. This gives a ground to assume that laser light irradiation in certain dosage and condition can improve functional abilities of cells. Future experiments are required to ascertain if stem cells respond to the laser light the same way.
The main finding on this study is that red and IR laser light can stimulate stem cell migration in vitro, and especially increase migration towards SDF-1α gradient. Stem cell ability to migrate towards tissues with higher SDF-1 concentration is one of the key mechanisms of stem cell homing. These results are giving ground to speculate that activation of stem cell migration can be one of the mechanisms of low level laser therapy. Taking into consideration that the combined of SDF-1 and laser irradiation had the strongest effect on stem cell homing, it would be reasonable to assume that this combination could be used in not only increasing the activity of stem cells but also in determining the main area of stem cell mobilization and homing. The current study did not aim to study the mechanisms of increased migration ability, which will be study in the future. But it is possible to suggest following explanation: laser irradiation can change the metabolism of stem cells, increase ATP production and so increase the migration, as well as up-regulate CXCR4 receptor expression or syntheses de novo. More studies are required to test if the laser light irradiation in vivo is able to make homing of transplanted stem cells to the area of damage more efficient, to check the influence of laser light on the mobilization rate of stem cells from bone marrow, to investigate if laser light can enhance functional abilities of stem cells. These studies would be desirable for better understanding of the mechanisms of laser therapy and for development of more effective methods of stem cell therapy.
References
1. Tuner J. and Hode L. Low Level Laser Therapy: Clinical Practice and Scientific Background, Prima Books, Grängesberg, Sweden, 1999.
2. Karu T. The Science of Low Power Laser Therapy, Gordon & Breach, London, 1998.
3. Baxter G.D. Therapeutic Lasers: Theory and Practice, Churchill Livingstone, London, 1994.
4. Simunovic Z., Ed. Lasers in Medicine and Dentistry, Vitgraf, Rijeka (Croatia), 2000.
5. Zhukov B.N. and Lysov N.A. Laser irradiation in experimental and clinical angiology (in Russian), Samara (Russia), 1996.
6. Kozlov V.I., et al. Bases of laser physio- and reflexo-therapy (in Russian), Zdorovje, Samara (Russia), 1993.
7. Paleev N.R. Ed. Phototherapy (in Russian), Meditsina, Moscow (Russia), 2001.
8. Skobelkin O. K. Ed. Application of low-intensive lasers in clinical practice (in Russian). Moscow, 1997.
9. Forrester J, Price M, Makkar R. Stem Cell Repair of Infarcted Myocardium. An Overview for Clinicians. Circulation. 2003;108:1139–1145.
10. Orlic D., Hill J., Arai A. Stem Cells for Myocardial Regeneration Circulation Research. 2002;91:1092.
11. Hodgson D., Behfar A., Zingman L.V., Kane G.C., Perez-Terzic C., Alekseev A.E., Puceat M., and Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol, August 1, 2004; 287(2): H471 - H479.
12. Ozbaran M., Omay S. B., Nalbantgil S., Kultursay H., Kumanlioglu K., Nart D., and Pektok E. Autologous peripheral stem cell transplantation in patients with congestive heart failure due to ischemic heart disease. Eur. J. Cardiothorac. Surg., March 1, 2004; 25(3): 342 - 350.
13. Brill A.G., Shenkman B., Brill G.E. et al. Blood irradiation by He-Ne laser induces a decrease in platelet responses to physiological agonists and an increase in platelet cyclic GMP. Platelets. 2000. Vol. 11. P. 87-93.
14. Mester A. Biostimulative effect in wound healing by continuous wave 820 nm laser diode. Lasers in Med Science, abstract issue July 1988, No. 289.
15. Gasparyan L.V. Stem cells and therapeutic effect of light irradiation (in Russian). Collection of abstracts of the 10th International Conference of Quantum Medicine, Moscow, 2003, pp. 43-44.
16. Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101.
17. Malouf NN, Coleman WB, Girsham JW, et al. Adult-derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol. 2001;158:1929–1935.
18. Donovan PJ, Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97.
19. Forrester J, Price M, Makkar R. Stem Cell Repair of Infarcted Myocardium. An Overview for Clinicians. Circulation. 2003;108:1139–1145.
20. Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757.
21. Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174.
22. Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779.
23. Yamaguchi J, Kusano K, Masuo O, at al. Stromal Cell–Derived Factor-1 Effects on Ex Vivo Expanded Endothelial Progenitor Cell Recruitment for Ischemic Neovascularization. Circulation. 2003;107: 1322–1328.
24. Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615-22.
25. Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833.
26. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638.
27. Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998; 393:591–594.
28. Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599.
29. Mohle R, Bautz F, Rafii S, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530.
30. Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34+ cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768.
31. Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360.
32. Salcedo R, Wasserman K, Young HA, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal derived factor-1α. Am J Pathol. 1999;154:1125–1135.
33. Damas J, Wæhre T, Yndestad A, et al. Stromal Cell–Derived Factor-1a in Unstable Angina. Circulation. 2002;106:36-42.
34. Pyrikova S.I et al. Effect of laser exposure on human seminal fluid (in Russian). Clinical and laboratory diagnosis. 1998;5:15-16.
Photobiomodulation of the Brain
Michael R. Hamblin and Yng-Ying Huang - 2019 (Publication) 4512This is Hamblin and Huang's best summary of PBM for treating the brain.
Original Source: https://books.google.com/books/about/Photobiomodulation_in_the_Brain.html?id=P0qiDwAAQBAJ&source=kp_book_description
A Practical Handbook: Laser Acupuncture
Volkmar Kreisel and Michael Weber - (Book) 4319This book is like a bible for laser acupuncture. It is the most detailed book on the subject that we have been able to find. It can be a little hard to get out because the publisher is in Germany. Dr. Weber operates a large clinic in Germany where he treat a wide variety of conditions. He also does training classes for acupuncturist and is a leader in the field of laser acupuncture. In addition to having a detailed explanation of how lasers stimulate the body, her provide some great general guidelines on the use of lasers and his book includes beautifully detailed protocols. Chapters in the book include 3 major sections: High-Tech Acupuncture with Laser Light, Practical Guidelines and Treatment Concepts. Within the treatment concepts are group of protocols for Orthopedics, Neurology, Psychosomatic disorders, Throat, Nose and Ear, Internal Medicine, Dermatology, Pediatrics, Gynecology ,Dental Medicine and Ophthalmology.
Original Source: http://www.coldlasers.org/lllt-books/
Are all the negative lllt studies really negative?
Tunér-Hode - 1998 (Publication) 4385This is an excerpt from the book "Low Level Laser Therapy" by Tunér-Hode, chapter 13. You will find the excerpt at the link below or here. This excerpt talks about how not all negative LLLT studies can necessarily say that LLLT does not work. The main problem being that the dose or wavelength was incorrect for the attempted treatment, leaving reasearchers with less than satisfactory results, in some cases laser parameters were not even recorded. While we must take negative studies seriously, it can be seen that once the majority of them have been examined that the attempted LLLT was simply being done incorrectly. You will find the excerpt broken up thusly:
- Are all the negative lllt studies really negative?
- "I heard it through the grapewine"
- Positive from negative
- Negative from negative
- Important parameters
- A. Wavelength
- B. Dose
- C. Power density
- Typical traditional laser instruments
- Dose development
- Pitfalls
- 1. Low outputs
- 2. Inclusion criteria
- 3. Lack of proper control groups
- 4. Therapeutic technique
- 5. Systemic effects
- 6. Tissue condition
- 7. Power density
- 8. Mixed parameters
- 9. The influence of ambient light
- 10. Premature conclusions
- 11. Meta-analyses
- Confusion between groups
Original Source: http://www.laser.nu/lllt/LLLT_critic_on_critics.htm
Light and Laser Therapy: CLINICAL PROCEDURES
Curtis Turchin, MA, DC - 2011 (Book) 4326This book introduces you to the science of Laser Therapy, starting with the history and basic physics of laser radiation, including things like:

- Lasers vs. LED's
- Measuring Wavelength
- The Electromagnetic Spectrum
- Depth of Laser Penetration
- Types of Laser Diodes
- Classification of Diodes
- Light Energy in Joules
- Pulsing or Frequency
- Treatment Parameters
- Light Absorbed
- Laser Safety
- Contraindications of Light Therapy
- Optimal Dose
- Calculating Output
- Dose and Time for Different Physical Qualities
You will find suggested treatments, and accompanying diagrams for the syndromes listed below:
Head and Face:
- Bell's Palsy
- Migraine Headache
- Sinusitis
- Temporomandibular Joint Syndrome (TMJ)
- Tension Headache
- Trigeminal Neuralgia
- Wrinkles
Spine and Pelvis:
- Cervicall Disc Herniation
- Cervical Stenosis
- Cervical, Thoracic, Lumbar Sprain/Strain and Neuritis
- Coccydynia
- Costochondritis
- Herniated Lumbar Disc or Annular Tear
- Lumbar Stenosis
- Pubic Symphysis Sprain
- Sacroiliac Sprain or Strain
- Spinal Hypermobility Syndrome
Systemic:
- Addiction to Cigarettes or Other Substances
- Ankylosing Spondylitis
- Arthritis
- Complex Regional Pain Syndrome or Reflex Sympathetic Dystrophy
- Fibromyalgia Syndrome (FMS)
- Herpes Zoster/Shingles and Post Herpetic Neuralgia
- Post Surgical Pain
- Wounds (Slow or Non-Healing)
Upper Body:
- Acromioclavicular (AC) Sprain or Laxity
- Biceps Tendinitis
- Biceps Tendon Strain
- Carpal Tunnel Syndrome
- DeQuervain's Tendinitis
- Dislocated Finger or Thumb
- Fractured Carpal, Metacarpal, or Phalange
- Fractured Clavicle
- Fractured Distal Radius or Casted Forearm or Hand
- Frozen Shoulder
- Ganglion Cyst of the Wrist
- Olecranon Bursitis
- Radial or Ulnar Neuritis
- Rotator Cuff Strain
- Shoulder Rheumatoid and Oseoarthritis
- Subacromial Bursitis
- Tennis and Golfer's Elbow
- Thumb or Finger Sprain
- Triceps Strain
- Wrist Flexor or Extensor Tendinitis
Lower Body:
- Achilles Tendinitis and Rupture
- Adductor Strain
- Anterior (ACP) and Posterior Compartment Pain (PCP)
- Anterior and Posterior Cruciate Ligament Injury
- Calcaneal Bursitis
- Calf Strain
- Dislocated Patella
- Hallux Valgus and Rigidus
- Hamstring or Ischiogluteal Bursitis and tendinitis
- Hamstring Strain
- Hip Sprain
- Interdigital Neuritis - Metatarsalgia - Morton's Neuroma
- Knee Contusion, Housemaid's Knee, Prepatellar Bursitis
- March or Stress Fracture
- Medial and Lateral Collateral Ligament Injury
- Meniscus Sprain/Strain
- Metatarsalgia - Thinning of the Fat Pad
- Osgood Schlatter Syndrome
- Osteochrondritis Dissecans
- Patellar Teninitis and Quadriceps Insertion Strain
- Patellofemoral Syndrome
- Peripheral Neuropathy (PN)
- Piriformis Syndrome
- Plantar Fasciitis
- Posterior Knee Swelling - Baker's Cyst
- Quadriceps Strain
- Restless Leg Syndrome or Leg Cramps
- Sesamoiditis
- Shin Splints
- Sprained Ankle
- Tarsal Tunnel Syndrome
- Tensor Fascia Lata and Iliotibial Band Syndrome
- Tibial or Fiula Stress Ftacture
- Trochanteric Bursitis
Original Source: http://www.coldlasers.org/lllt-books/
Laser Therapy and Laser Puncture in Dogs and Cats
Anja Füchtenbusch and Peter Rosin - 2012 (Book) 4327In the book "Laser Therapy and Laser Puncture in Dogs and Cats", you will find acupuncture points and meridians for cats and dogs, with accompanying diagrams in the appendix. This book will go over using your laser therapy system, and determining treatment times and plans; as well as protective measures, contraindications, and side effects. The bulk of the book is about Finding the right treatment plan, and is broken into color coded sections thusly:
- Guidelines
- Wounds, Scars, Disturbance Fields
- Pain
- Locomotor System
- Repiratory Tract
- Gastrointestinal Tract
- Bladder, and Kidneys
- Liver
- Spleen, and Pancreas
- Vessels
- Metabolism, and Detoxification
- Skin
- Immune System
- Psyche
- Other Disorders
Original Source: http://www.coldlasers.org/lllt-books/
Quantum Acupuncture: The Next Level
Ronald Henery DC, ND, FIAMA - 2011 (Book) 4331This book consists mostly of diagrams and charts explaining the different points and meridians on the human body.

Five Elements and the Chakras
- five elements
- the nurturing cycle
- the control cycle
- beyond the five elements
- 24-hour horary cycle
- chakra system in meridian therapy
The Meridians
- the meridian network
- the relation between meridians and their organs
- chapter 2 overview
- heart
- small intestine
- bladder
- kidney
- pericardium
- triple heater
- gallbladder
- liver
- lung
- large intestine
- stomach
- spleen
- governor vessel
- conception vessel
Point Group Use in Diagnosis and Treatment
- tonification and sedation points
- master points
- source points
- luo points
- accumulation points
- command points
- alarm points
- bladder association points
- ifluential points
- transporting points
- the jing-well points
- the spring points
- the stream points
- the river points
- the sea points
- lower he-sea points
- meeting points
- ma dan-yang points
- vitality collapse points
- entry-exit points
- gohst points
- pulse points
- mensturation points
- pregnancy points
- musculo-tendendo points
Key Points on the Meridians
- heart meridian
- small intestine meridian
- bladder meridian
- kidney meridian
- pericardium meridian
- triple heater meridian
- gallbladder meridian
- liver meridian
- lung meridian
- large intestine meridian
- stomach meridian
- spleen meridian
- governor vessel
- conception vessel
Point Location
- heart
- small intestine
- bladder
- kidney
- pericadium
- triple heater
- gallbladder
- liver
- lung
- large intestine
- stomach
- spleen
- governor vessel
- conception vessel
Original Source: http://www.coldlasers.org/lllt-books/
Laser Phototherapy Clinical Practice and Scientific Background
Lars Hode and Jan Tunér - 2014 (Book) 4328This book is one of the most comprehensive resources for European style laser therapy.
This book covers an astonishing amount of information in its near thousand pages, everthing from basic laser physics to dental, and veteranary useage. Here are some of its contents:

- Basic Laser Physics
- physics
- energy
- radiation
- wavelength and frequency
- photon energy
- the elecromagnetic spectrum
- the optical reigon
- radiation risks
- can electromagnetic radiation cause cancer
- protective mechanisms
- light
- the optical spectrum
- light sources
- various sources of radiation
- natural sources of radiation
- man-made light sources
- the light emmiting diode (LED)
- flash lamps
- the laser
- laser design
- practical lasers
- the properties of laser light coherence
- interference
- laser beam characteristics
- polarisation
- output power
- continuous and pulsed lasers
- the peak power value
- average power output
- power density
- light distribution
- beam divergence
- collimation
- risk of eye injury
- decisive factors in the risk of eye injury
- the laser instrument
- properties of some laser types
- description of common surgical laser types
- the CO2 laser (carbon dioxide laser)
- carbon dioxide lasers in surgery
- carbon dioxide lasers in dental applications
- the Nd:YAG laser
- Nd:YAG lasers in surgery
- Nd:YAG lasers in dentistry
- erbium lasers in dentistry
- "strong" diode lasers in dentistry
- the KTP laser
- Q-switching
- Theraputic Lasers
- the first generation 1975-85
- the second generation 1985-95
- the third generation 1995-2005
- the fourth generation 2005 and onwards
- what is a good laser therapy instrument
- the basic instrument
- sales tricks
- high power-low power
- laser or LED
- high or low price
- penetration of light into tissue
- "a story of a young scientist"
- the wavelength
- how deep does light penetrate into tissue?
- Biostimulation
- history
- a few words on mechanisms
- photoreceptors
- what parameters to use
- laser parameters
- whitch wavelength?
- output power
- average output power
- power density
- energy density
- the dose
- treatment dose
- calculation of doses
- dose ranges
- calculation of treatment time for a desired dose
- "reay reckoner"
- dose per point
- pulsed or continuous light
- pulse repetition rate (PRP)
- patient parameters
- treatment area
- treatment intervals
- pre- or postoperative treatment
- treatment method parameters
- local treatment
- shallow problems
- deeper problems
- treating inside the body
- systemic treatments
- acccupuncture
- trigger points
- spinal processes
- dermatome
- blood irradiation
- irradiation of lymph nodes
- irradiation of ganglions
- combo treatment
- interaction with medication
- other considerations
- what about collimation?
- depth of penetration, greatest active depth
- factors that reduce penetration
- tissue compression
- how deep does the light penetrate?
- laser light irradiation through clothes
- the importance of tissue and cell condition
- the importance of ambient light
- in vitro/ in vivo
- laser therapy with high output lasers
- laser therapy with carbon dioxide lasers
- laser therapy with Nd:YAG lasers
- laser therapy with ruby lasers
- laser therapy with Er:YAG lasers
- laser therapy with surgical diode lasers
- risks and side effects
- the importance of correct diagnose
- cancer
- cytogentic effects?
- a false picture of health
- tiredness
- pain reaction
- do high doses of laser therapy damage tissue?
- is it only an effect of temperature?
- protection against radiation injury
- how to measure effects of laser therapy
- thermography
- magnetic resonance imaging
- high resolution digitized ultrasound B-scan
- tensile strength
- other objective methods
- does it have to be a laser?
- FDA (Food and Drug Administration)
- how well documented?
- confused?
- the funding research
- as time goes by
- Medical indications
- who and what can be treated?
- acne
- allergy
- antibiotic resistance
- arteriosclerosis
- arthritis
- asthma
- blood preservation
- blood pressure
- bone regeneration
- burning mouth syndrome
- cancer
- cardiac conditions
- carpal tunnel syndrome
- cerebral palsy
- crural and venous ulcers
- delayed onset muscular soreness (DOMS)
- depression, psychosomatic problems
- diabetes
- duodenal/gastric ulcer
- epicondylitis
- erythema multiform major
- fibrositis/fribomyalgia
- headache/migraine
- heamorrhoids
- herpes simplex
- immune system modulation
- inflammation
- inner ear conditions
- laryngitis
- lichen
- low back pain
- mastitis
- microcirculation
- morbus sluder
- mucositis
- muscle regeneration
- mycosis
- nerve conduction
- nerve regeneration and function
- oedema
- ophthalmic problems
- pain
- periostitis
- plantar fasciitis
- salivary glands
- sinuitis
- spinal cord injuries
- snake bites
- sports injuries
- stem cells
- stroke, irradiation of the brain
- tendinopathies
- tinnitus, vertigo, meniere's disease
- tonsillitis
- trigeminal neuralgia
- thrombophlebitis
- tuberculosis
- urology
- warts
- wiplash-assosiated dissorders
- vitiligo
- womens' health
- wound healing
- zoster
- idications in the pipeline
- alzheimer's disease
- botox failures
- cellulites
- cholesterol reduction
- complex reigonal pain syndrom (CRPS)
- eczema
- erectile dysfunction
- familiar amyotrophic lateral sclerosis (FALS)
- glomerulonephritis
- obesity
- orofacial granulomatosis
- Parkinson's disease
- post-mestrual stress
- pemphigus vulgaris
- sleeping disorders
- withdrawal periods
- wrinkles
- consumer lasers
- Dental LPT
- the dental laser literature
- on which patients can LPT be used?
- dental indications
- alveolitis
- anaesthetics
- aphthae
- bleeding
- bisphosphonate related osteonecrosis of the jaw
- caries
- dentitio dificilis (pericoronitis)
- endodontics
- extraction
- gingivitus
- herpes zoster
- hypersensitive dentine
- implantology
- leukoplakia
- lingua geographica (glossitis)
- lip wounds
- nausea
- nerve injury
- orthodontics
- mild dental pain
- paediatric dental treatment
- periodontics
- prosthetics
- root fractures
- secondary dentine formations
- temperature caveats
- toemporo-mandibular disorders (TMD)
- TMD and endodontics
- other dental laser applications
- dental pohoto dynamic therapy
- composite curing
- deminerallisation
- tooth bleaching
- caries detection
- lasers as a diagnostic tool
- case reports
- Non Coherent Light Sources
- Veterinary Use
- case reports
- Contra Idications
- pacemakers
- pregnancy
- epilepsy
- thyroid gland
- children
- cancer
- haemophilia
- irradiation of the brain
- radiation therapy patients
- diabetes
- tatoos
- light sensitivity
- Coherence
- the role of coherence in laser phototherapy
- itroduction
- summary
- Dose and Intensity
- basics about energy
- output power
- power density
- the laser beam
- the laser probe
- pulsed lasers
- energy density
- treatment dose
- the dose does not demend on the intensity
- dose per point
- more about treatment technique
- The Mechanisms
- are biostimulative effects laser specific?
- is it possible to prove that laser therapy doesn't work?
- comparisons between coherent and non-coherent light
- what is the importance of the length of coherence
- hode's hamburger
- hode's big burger
- abrahamson's apple
- moonlight
- how deep does light penetrate tissue?
- bright light phototherapy
- similarities and differences
- possible primary mechanisms
- polarisation effects
- what characterises the light in a laser speckle
- porphyrins and polarised light
- cell cultures and tissue have different optical properties
- tthe effect of heat development in the tissue
- macroscopic heating
- the microscopic heat effect
- mechanical forces
- excitation effects
- primary reactions due to excitation
- secondary reactions due to cell signaling
- flourescence-luminescence
- multi-photon effects
- llasting effects in tissue
- non-linear optical effects
- opto-acoustic waves
- secondary mechanisms
- effects on pain
- effects on blood circulation
- stimulatory and regulatory mechanisms
- effects on the immune system
- other interesting possibilities
- summary of mechanisms
- diagnostics with therapeutic lasers
- photodynamic therapy - PDT
- other medical uses of lasers
- A Guide for Scientific Work
- methodology of a trial
- parameters
- technical parameters
- treatment parameters
- medical parameters
- closer description of the technical parameters
- name of instrument (producer)
- laser type and wavelength
- laser beam characteristics
- number of sources
- beam delivery system
- output power
- power density at probe aperture
- calibration of the instrument
- closer description of the treatment parameters
- treatment area
- dose: energy density
- dose per treatment and total dose
- intensity: power density
- treatment method
- treatment distance (spot size), type of movement, scanning
- sites of treatment
- number of treatment sessions
- frequency of treatment sessions
- closer description of the medical parameters
- description of the problem to be treated
- patients (number, age, sex)
- exclusion criteria
- inclusion criteria
- condition of patient
- pre-, parallel-, or post-medication
- treated with other methods before
- drop-out rates
- follow up
- outcome measures
- statistical analysis
- economy
- gallium-alluminium and all that
- recommendations of WALT - the world assosiation for laser therapy
- The Laser Phototherapy Literature
- the importance of reporting all laser parameters - even in the abstract
- diclofenac, dexamethasone or laser phototherapy?
- another pithole in LPT research
- database of abstracts of reviews of effects (DARE)
- the wound healing contradiction
- wikipedia
- poor documentation - compared to what?
- LPT equipment and the future
- english language books od LPT:
- books in other languages, with ISBN
- laser phototherapy journals
- information for your patient
Original Source: http://www.coldlasers.org/lllt-books/
A Practical Handbook Laser Acupuncture Successful Treatment Concepts
Volkmar Kreisel and Michael Weber - 2012 (Book) 4330This book is our top recommendation for any acupuncture style cold laser treatment protocols. It is beautifully written and illustrated.

High-Tech Acupuncture with Laser Light
- an equisite light therapy
- biostimulation
- light can heal
- primary stimulation effects
- secondary simulation effects
- is ther optimum stimulation
- laser acupuncture
- high-tech and tradition
- laser ear acupuncture
- is there an optimum dose
- resonance therapy
- what is resonance therapy
- explanatory models
- laser frequencies and those who discovered them
- resonance theapy on the ear
- additional methods and synergisms
- suplementary acupuncture methods
- special applications
- synergisms
- laser types
- laser types by wavelength
- laser types by type of signal
- laser types by form of application
- laser classes
Practical Guidelines
- point localization
- selecting the frequency
- recommended doses
- laser acupuncture: doses and treatment time with laser pen and laser needle
- area therapy: dose and treatment time with laser shower and dermaspot
- important information regarding therapy plans
- containdications and side effects
Treatment Concepts
- orthopedics
- achillodynia
- arthitis, idiopathic juvenile (pediatric rheumatism)
- arthritis, rheumatoid (chronic polyarthritis)
- arthritis urica (gouty arthritis)
- aseptic osteonecrosis
- bakers cyst (popliteal cyst)
- slipped disk (spinal disk herniation)
- bursitis
- chrondophathia patellae
- coxarthosis (arthosis of th hip)
- CRPS (complex regional pain syndrome, Sudeck's disease, reflex dystrophy)
- epicondylitis humeri
- exostosis (bony outgrowth)
- heel spur (calcaneal spur)
- fibromyalgia
- gonarthosis (arthosis of the knee joint)
- hallux valgus (hallux rigidus, bunion)
- cervical spine syndrome
- sacroiliac joint blockage (SIJ blockage)
- capsular ligament injury
- lumbosciatica (sciatica syndrome, irritation of the nerve root)
- lymphatic edema, postoperative
- metataralgia
- muscle fiber rupture (traumatic myopathy)
- Myofascial pain syndrome
- shoulder-arm syndrome
- spinal canal stenosis
- wound healing disorder
- neurology
- carple tunnel syndrome CTS (median nerve compression syndrome)
- cephalgia
- facial paresis
- migraine
- multiple sclerosis MS (encephalomyelitis disseminata)
- paresis (incomplete paralysis)
- Parkinson's syndrome (Parkinson's disease)
- phantom pain
- polyneuropathy
- restless leg syndrome RLS
- transient ischemic attack TIA (stroke)
- psychosomatic disorders
- anorexia nervosa
- burnout syndrome
- depression
- jet lag (dysrhythmia)
- concentration disorders
- addictions - alcohol abuse
- addictions - nicotine abuse
- throat, nose and ear
- otitis media (inflammation of the middle ear)
- parotitis
- acute sinusitis
- chronic sinusitis
- tinnitus
- tonsillitus (angina tonsillaris)
- internal medicine
- allergic disorders - basic laser desensitization
- allergic disorders - allergic exanthema
- allergic disorders - hay fever
- allergic disorders - food allergies
- angiopathies - chronic venous insufficiency CVI
- angiopathies - hemorrhoids
- angiopathies - raynaud's disease
- angiopathies - thrombophlebitis
- gastrointestinal disorders - ulcerative colitis
- gastrointestinal disorders - gastritis
- gastrointestinal disorders - hepatitis
- gastrointestinal disorders - crohn's disease
- lung disorders - bronchial asthma
- lung disorders - acute bronchitis
- lung disorders - chronic bronchitis
- lung disorders - COPD (chronic obstructive pulmonary disease)
- metabolic disorders - diabetes mellitus
- dematology
- acne (acne simplex)
- atopic eczema / neurodermatitis
- hyperhidrosis
- psoriasis
- seborrjeic eczema
- pediatrics
- adenoids (adenoid vegetations, polps, palatine tonsil)
- attention deficit hyperactivity syndrome ADHS
- attention deficit syndrome (concentration disorder)
- abdominal pain, functional
- chronic bronchitits
- three months' colic (regulation disorder / infant crying)
- enuresis nocturna (bedwetting)
- whooping cough (petussis)
- tympanic effusion (tubal catarrh)
- obesity (adipositas)
- underweight (growth disorder)
- cerebral paresis (cerbral palsy)
- gynaecology
- mastitis (inflammation of the mammary glands)
- PMS (postmenstral syndrome)
- morning sickness (hyperemesis gravidarium)
- dental medicine
- stomatitis/gingivitis/aphtea
- tooth extractions
- bleeding gums
- toothache
- ophthalmology
- age-related macular degeneration AMD
- central serous chorioretinopathy (central serous retinitis)
- glaucoma
- conjuctivitis
- retinitis pigmentosa
- dry eyes (sicca syndrome)
Original Source: http://www.coldlasers.org/lllt-books/
Handbook of Photomedicine
Michael R. Hamblin, PhD and Ying-Ying Huang, MD - 2014 (Book) 4333The Handbook of Photomedicine includes info on all types of medical lasers used in medicine including LLLT, surgical usage and photodynamic therapy (PDT).

History and Fundamentals
- introduction: historical vignettes from the feild of photomedicine
- history and fundamentals of lasers and light sources in photomedicine
- light-tissue interactions
- history and fundamentals of photodynamic therapy
- history and fundamentals of low-level laser therapy
Diseases Caused by Light
- uv effects on the skin
- photocarcinogenesis nonmelenoma skin cancer
- autoimmune photodermatoses
- photoaging
- uvr-induced immunosurpression
- the porphyrias
- photoprotection
- botanical antioxidants for photochemoprevention
- reversal of DNA damage to the skin with DNA repair liposomes
- climate change and ultraviolet radiation exposure
- photochemistry and photobiology of vitamin D
Ultraviolet Phototherapy
- phototherapy for psoriasis
- PUVA therapy
- extracorporeal photopheresis
- ultraviolet C therapy for infections
Photodynamic Therapy (PDT)
- recent advances in developing improved agents for photodynamic therapy
- 5-aminolevulinic acid and its derivatives
- genetically encoded photosensitizers: structure, photosensitization mechanisms, and potential application to photodynamic therapy
- light dosimetry for photodynamic therapy: basic concepts
- multimodality dosimetry
- cell death and PDT-based photooxidative (phox) stress
- vascular and cellular targeted PDT
- photodynamic therapy for increased delivery of anticancer drugs
- targeting strategies in photodynamic therapy for cancer treatment
- enhancing photodynamic treatment of cancer with mechanism-based combination stratagies
- nanoparticles for photodynamic cancer therapy
- drug delivery stratagies for photodynamic therapy
- antimicrobial PDT fo clinical infectious diseases
- PDT and the immune system
- detection of bladder cancer by fluorescence cystocopy: from bench to bedside the hexvix story
- photochemical internalization: from bench to bedside with a novel technology for targeted macromolecule therapy
- the story of tookad: from bench to bedside
- photodynamic therapy in ophthalmology
- photodynamic therapy in dermatology
- photodynamic therapy in the gastrointestinal tract
- photodynamic application in brain tumors
- photodynamic therapy for malignant pleural disease
- clinical photodynamic therapy in the Chinese region
- photodynamic therapy and fluorescent diagnostics in the Russian federation
Low-Level Laser (Light) Therapy (LLLT)
- chromophores (photoacceptors) for low-level laser therapy
- low-level laser therapy signaling pathways
- irradiation parameters, dose response, and devices
- low-level laser therapy: clearly a new paradigm in the management of cancer therapy- induced mucositis
- low-level laser therapy for wound healing
- low-level laser therapy in the treatment of pain
- low-level laser therapy in arthritis and tendinopathies
- low-level laser therapy and LED therapy on muscle tissue: preformance, fatigue, and repair
- low-level laser therapy for stroke and brain disease
- low-level light therapy for nerve and spinal cord regeneration
- low-level laser therapy in dentistry
- low-level laser therapy and stem cells
- low-level light therapy for cosmetics and dermatology
Surgical Laser Therapy
- laser and intense pulsed light treatment of skin
- therapeutic uses of lasers in eye care
- lasers used in dentistry
- lasers used in urology
- lasers used in otolaryngology
- laser treatment to nanoparticles for theranostic applications
- laser imminutherapy
- tissue repair by photochemical cross-linking
Other Phototherapies an Future Outlook
- optical guidance for cance interventions
- phototherapy for newborn jaundice
- biological evidence of the efficacy of light therapy in psychiatric disorders
- future developments in photomedicine and photodynamic therapy
Original Source: http://www.coldlasers.org/lllt-books/
Performance Chiropractic and Wellness: The Complete A-Z Manual for Low Level Laser Therapy 5th edition
Jerome Rerucha B.S., C.S.C.S., D.C. - 2015 (Book) 4332Dr Rerucha is on the cutting edge at documenting how different pulsing frequencies can be used for different stilulatory effects. He works mainly with Erchonia.

The Biological Basics of Low Level Laser Light Therapy
- summary
- introduction
- Alexander Gurwitsch: cells emit light
- non-linear dynamics
- introducing quantum physics
- itroduction to quantum biology
- quantum coherence in biology
- biological coherence and the sensitivity of living systems
- Fritz Albert Popp: biophotons
- Guenther Albreecht-Buehler: cells respont to light
- Mae-Wan Ho: visualizing coherence
- conclusions
Therapeutic Laser Applications
- how does low level laser therapy work?
- what are the advantages over other modes of therapy?
- cliniclal use of low level laser therapy
- abstract submitted to laser and surgury medicine
- background and objective
- methods
- results
- conclusion
- safety considerations
- eye considerations
- pace makers and other implanted devices
- pregnancy
- excessive toxicity
- preface to treatment section
Nerver Roots
- flexion and extension
- lateral flexion
- rotation
- MRT (muscle response testing) through ROM of cervical spine
- shoulder
- neurological level
- C5
- C6
- C7
- C8
- T1
- S1
- L5
- L4
- L3
- L3-L5
- L2-L4
- L1-L3
- low back
Top Ten Laser Protocols
- organ / glands / tissue
- acute injury (shock)
- pain
- lymphatic protocol
- detox protocol
- immune protocol
- hormone protocol
- basic cranial nerve
- tissue memory
- trauma preparation protocol
A-Z Laser Protocols
- abdominal cramping
- abdominal inflammation/pain
- abrasions
- abscess
- achilles tear / strain (partial only; not rupture)
- acidosis (hyperacidity
- acid reflux
- acne
- acute injury
- adenoids
- (ADD) atention deficit disorder and hyperactivity disorder (ADHD)
- Addiction
- addison's disease
- adhesions
- adhesive capsulitis
- adrenal
- aids
- allergies
- alopecia
- alpha waves
- alzheimer's
- amenorrhea
- amoebas
- amyotrophic lateral sclerosis / lou gehrig's disease / motor neuron
- amnesia
- anemia
- anger
- angina
- anosmia (loss of smell)
- anxiety appendicitis
- arrhythmias
- arteries / arteriosclerosis
- arthritis
- asthma
- ataxia
- athlete's foot
- atrophy
- backache / back pain
- bacteria
- bed sores
- bedwetting
- bell's palsy
- beta waves
- bites
- bladder
- bleeding gums
- bloating
- blood pressure (high)
- blood pressure (low)
- blood sugar balance
- boils
- bone
- bowel
- bradycardia
- brain
- breast augmentation
- bronchitis
- bruises
- buerger's disease
- bunions
- burns
- burns (second degree)
- bursitis
- calcium deposits or formations
- candida
- canker sores
- capsulitis
- carpal tunnel syndrome
- cartilage
- cataracts
- chemical peels / resurfacing
- chest pain
- chicken pox (herpes zoster / varicella)
- cholecystitis
- cholelithiasis
- chronic fatigue
- chronic pain
- circulation
- cirrhosis
- cold sores (herpes simplex 1)
- colds and flu
- colitis
- concussion
- confusion
- congestion
- congestive heart falure (CHF)
- conjunctivitis (pink eye)
- costipation
- cramps (muscle)
- cranial nerves (general)
- cranial nerves VIII
- crepitus
- crohn's disease
- cuts
- cushing's syndrome
- cytomegalovirus (herpes syndrome V)
- deer tick
- delta waves
- depression
- dermatitis
- detoxification
- diabetes
- diabetic neuropathy
- diabetic ulcers
- digestion
- dim vision
- disc herniation
- dizziness
- dupuytren's contracture
- dyslexia
- ear ache
- ear infection
- eczema
- edema
- emotional stress
- emphysema
- emulsification of fat
- endometriosis
- epistaxis
- epstein - barr virus
- esophagitis
- exercise recovery
- eye conditions
- facet syndrome
- facial paralysis
- fever
- fever blisters
- fibromyalgia
- flu
- food intolerance
- food poisoning
- foot fungus
- fracture
- fungus
- gait
- gallbladder (general)
- gallbladder (stones)
- ganglion cyst
- general musculoskeletal
- gerd
- gingivitis
- glaucoma
- goiter
- gout
- gums
- headache
- heart
- heartburn
- hearing difficulty
- hemorrhoids
- hepatitis A
- hepatitis B
- hepatitis C
- hernia
- herpes simplex
- herpes zoster (chickenpox / varicella)
- HIV
- hives
- hoarseness
- hormone balance
- hot flashes
- human papilloma virus (HPV)
- hyperactivity
- hyper/hypo-tension
- hyper/hypo-thyroid
- hyper/hypo-gycemia
- impotence
- immune enhancement
- incontinence
- indigestion
- infection
- inflammatory bowel disease
- inflammation
- influenza
- injuries
- insect bites
- irritable bowel syndrome
- ischemia
- jaundice
- joints
- keloid
- kidney
- kidey stones
- large intestine
- laryngitis
- ligament
- liposuction
- liver (balace and support)
- loss of smell (anosmia)
- loss of taste
- low back pain
- lungs
- lyme disease
- lymphadentis
- lymphatic
- macular degeneration
- memory problems
- meniere's disease
- meniscus sprain (grade 1)
- menopause
- mensturation
- mental fatigue
- meridian balance 15
- migraine
- motion sickness
- multiple sclerosis
- muscle
- muscle spasm
- myocardial inrarction
- nerve root
- neurogenic inflammation
- neuropathy
- nervousness
- nose bleed
- numbness
- nystagmus
- ocular motility disorders
- ocular nerve
- olfactory nerve
- osgood-schlatter disease
- otitis
- pain
- pain (chronic)
- pain (general)
- injury related pain (localized)
- pain (acute injury)
- pancreas
- parasite
- parasympathetic facilitazation
- paresthesia (numbness)
- periodontal disease
- pink eye (conjunctivitis)
- plantar fasciitis
- pneumonia
- polycystic kidney diseases
- polycystic ovary
- post operative scar revision
- post operative wound healing / pain
- post traumatic stress disorder (PTSD)
- postnasal drip
- premenstral syndrome (PMS)
- pre set head PL-touch
- pre-op
- prostate
- psoriasis
- punctures
- rash
- reflex sympathetic dystrophy (RSD)
- renal problems
- respiratory problems
- restless leg syndrome
- retinitis pigmentosa
- rheumatism
- ringworm
- road rash
- scar tissue
- sciatica
- sedation
- seizures
- shingles
- sinusitis
- skin
- sleep apnea
- small intesine
- smell - lack of
- sore throat
- soreness
- spasm
- spider veins
- spleen
- sprains
- spurs
- standars (neurological) setting
- stanard (up-regulation) setting
- staph infection
- stings
- stomach ulcer
- strep infections
- stress
- stroke
- sty
- subluxation
- sunburns
- swimmer's ear
- swollen ankles
- sympathetic calming
- tachycardia
- taste - lack of
- teeth
- tendonmyopathy (tendonitis)
- tension headaches
- theta waves
- thoratic outlet syndrome
- throat
- thrush
- thyroid (hyper)
- thyroid (hypo)
- tinnitus
- TMJ
- toenail fungus
- tonsilitis
- toothache
- ulcer
- ulcerative colotis
- up-regulation
- urinary tract infection
- varicose veins
- veins
- venereal warts
- viral infections
- voice
- vomiting
- water retention
- watery discharge from eye
- warts
- wounds
- yeast
Original Source: http://www.coldlasers.org/lllt-books/
No Cure from LiteCure
Jan Tunér, Mar 22, 2014 - Annals of Laser Therapy Research (Publication) 4402This article from Jan Tuner talks about LiteCure's horrible marketing, how 980nm is really bad and how Class 4 lasers are too powerful and often misused. They recommend 905nm for deep penetration.
More Lies and Subterfuge from the World of Class IV Laser Therapy
By Jan Tunér
The US laser manufacturer LiteCure (a.k.a. Companion/Pegasus for veterinary version) belongs to a group of laser manufacturers that confuse customers and let consumers pay a high price for something that they do not need. LaserAnnals has previously addressed the so-called Class IV lasers for LPT in general and in a few cases mentioned this particular culprit LiteCure. In this article, we will make a closer check on the credibility and ethics of this company.
Marketing is generally a way of stretching the truth or at least highlighting potential benefits of a product without mentioning the drawbacks. Not very ethical but more or less what consumers expect. Sheer lying is a bit different, and LiteCure uses blatant lies in its marketing. Let us see the first lie:
Lie #1. LiteCure originally claimed that 980 nm has a much better penetration than 808 nm, and that the very high output of their lasers improves the penetration. The illustration below is from their early attempts at marketing the supposed benefits of their device:
Anyone with some basic knowledge about tissue optics knows that 980 nm has a poor penetration due to absorption by water and lipids, and that 808 nm (the illustration actually states 880 nm, but this is not a commonly-used laser wavelength so we assume this was another error…) actually is in an optical window where penetration through skin is optimal. Using very high power with 980 nm doesn’t increase penetration considerably, but instead causes more light to be absorbed superficially more quickly, leading to heat generation. And LPT is not based upon heat but upon stimulation!
Knowledgeable scientists, experienced clinicians and other manufacturers were quick to criticise, however, and to call LiteCure out on this lie, and over time LiteCure has responded by adding the deeper-penetrating 810 nm wavelength to their products, and by modifying the image, as follows:
Although a step in the right direction, even this illustration is still misleading and, basically, incorrect: The effective depth of laser irradiation does not increase over time.
Further to that, the “effortless” non-contact technique causes considerable energy loss by reflection and backscatter – together, remittance, which has been measured at upwards of 80% from bare skin (Al Watban, 1996) – and up to 100% energy loss due to absorption within animal hair/fur. This is hardly “efficient”!
The truth is the opposite to what their sales claims try to tell: A 0.5 W 808-810 nm Class 3B laser actually has a superior ability to penetrate into the body, whereas a 10.0 W 980 nm Class 4 has limited ability and also causes more problems with regards to heat generation. And, as the lower-powered Class 3B device may be applied in contact with the skin directly over the pathological tissue, and held steady for the necessary time to deliver the appropriate amount of energy, it is also significantly more efficient, accurate and safe.
The problem is that their consumer group is rather ignorant about LPT basics and swallow the bait. Fortunately for LiteCure, very high energies are bio-inhibitory and have a temporary pain relieving effect. This is an impressing effect when demonstrated. The downside of the procedure is that the needed reduction of an inflammatory process in inhibited and so is the body’s ability to regenerate itself. This is what is called “a sales trick”.
Lie #2. In its advertising material the LiteCure company writes: “World renowned Laser Therapy Experts, Jan Tunér and Lars Hode have indicated the advantages of high power laser therapy. The (research) literature supports the hypothesis that higher power density yields better clinical results.”
This is similar to the way the devil reads the bible. The above conclusion follows a part of our book where the remarkably low powered lasers on the Canadian market in the ‘90s is discussed. The vast majority of the lasers used were HeNe 1-2 mW and GaAlAs 5-30 mW. So the 400 mW lasers that had just arrived on the market at that time seemed to have a new potential – and they had.
Continued reading of our book reveals that high energies probably will have a better effect on pain conditions but probably not on superficial conditions such as wound healing. In fact, the discussion following the text about “high power” strongly modulates their usefulness.
This text appeared initially in the 2002 book “Low level laser therapy – clinical practice and scientific background”. In following versions of this book, the text has been modified and becomes more critical of extreme energies. And believe me, the next one will be even more critical, to avoid any misunderstandings.
Read my lips: “Tunér and Hode do not recommend 15 W Class IV lasers, not even 5 W!”
An appropriately configured and applied Class 3B device can do all that we need, and if you want to reach deep targets the 904 nm superpulsed GaAs is the best tool!
LiteCure type of science
Recently a LiteCure research paper on fibromylaglia (FM) was published:
Panton L, Simonavice E, Williams K, Mojock C, Kim JS, Kingsley JD, McMillan V, Mathis R. Effects of Class IV laser therapy on fibromyalgia impact and function in women with fibromyalgia. J Altern Complement Med. 2013 May;19(5):445-52.
FM is a devastating condition and LPT is probably a viable option to use, especially since other therapies are rather ineffective and life-long intake of painkillers not a viable option, with the side effects in mind. The study by Panton is obviously performed by a competent team of medical experts, but it seems they have “been taken for a ride” by the LiteCure company. The overall effect of the laser treatment was modest, but had some effects.
So let us have a look on this paper…
For the laser group, treatment was rendered utilizing a LCT-1000 (LiteCure LLC, Newark, DE) solid-state GaAlAs laser delivering a continuous-wave, dual-wavelength laser with 20% 810 nm, and 80% 980nm at 10 W. Each 56.45 cm2 treatment point was treated with laser at 10.63 J/cm2 and warm air utilizing a grid scanning technique to avoid overheating tissue. Participants were instructed to expect some warmth but that the treatment should not burn and to provide verbal cues if the treatment spots became excessively warm. Each treatment point was treated for exactly 60 seconds for a total of 600 J per point, for a total daily treatment dose of 4200 J. The dual wavelength was used for two reasons: (1) this is what is commercially available and (2) two wavelengths allow for treatment in patients with different skin colours since different melanin concentrations will absorb light differently. Both wavelengths are in the accepted therapeutic window. The sham treatment consisted of 60 seconds of warm air alone over the seven tender points.
Now, let us try to make some sense about this study:
a. The cause of FM is not known, but it is manifested by painful bodily points. If pain were a separate biological unit, smashing it with a sledge hammer might be useful. But there is probably more to it, like peripheral neural sensitisation and inflammation. 600 J (!) is given to each point and this is a very high and quite inhibitive energy. And a “point” is declared to be 56.45 cm2. This is rather an area. But by spreading out the light over a large area, the dose becomes 10.63 J/cm2. Such a dose appears to be reasonable, but the energy is not.
b. The paper says: Like the IIIB lasers, recently developed Class IV therapeutic lasers use diffuse light at wavelengths in a therapeutic window that allow penetration of the light deep into the tissue. True, but these lasers do not penetrate deeper than the Class IIIB/3B lasers, so this is a deliberately misleading statement. Further, Class IV/4 therapeutic lasers are not exactly “recently developed”: The defocused beams of Class IV/4 surgical lasers have been used for therapy for equally as long as Class IIIB/3B devices. And the first commercially-available dedicated Class IV/4 therapeutic lasers came on the market in Europe during the ‘90s – which, of course, contradicts the claims by LiteCure and others that Class IV/4 laser therapy is new improvement of Class IIIB/3B. As they are now, these earlier Class IV/4 therapeutic lasers were very expensive and inefficient, and proved no more effective than the already-available lower-powered lasers, so their use did not flourish until the marketing machine took hold in the USA.
c. The paper says: This development has led to the use of Class IV lasers to treat a variety of conditions including skin lesions(24,25), acute soft-tissue injuries (26), and chronic pain syndromes (27) such as FM. In fact, the references 24-27 are not related to the use of “Class IV” LPT lasers at all! This is a technique used often by LiteCure and other marketers of high-powered Class IV therapeutic lasers, banking on the fact that the casual reader will not follow through and actually read the referenced studies.
d. The paper says: There are only a few studies that have used laser therapy to treat pain (16,17,27,37,38). What about 125 published RCTs? If changed to “FM pain”, this is a more valid statement. And one of the most frequently quoted papers on FM and LPT (Gür et al.) used 2 J per point and with better results.
e. The paper says: Studies suggest that Class IV lasers have a beneficial analgesic and anti-inflammatory effect in humans (47-50). No, they don’t! All four papers to which they’ve referred are on Class 3B!
f. Previous studies on FM and LPT have been using considerably lower energies, so the reason for increasing these by a factor 100 seems to have but one background: To prove the superiority of the manufacturer’s product. However, the clinical outcome of this paper was not better than those where is Class 3B lasers have been used.
And let’s address another niggling falsehood: There is no such thing as “Class IV technology”!! 499 mW is Class 3B, 501 mW is Class IV. This is no “technology”. Laser classification is simply related to the relative risk posed by the power, wavelength and distribution of the laser emission!
The manufacturers of the Class IV lasers used in LPT have sponsored a small number of clinical studies. They all contain considerable flaws and even lies and are far from convincing. But they do contribute to the general confusion and are an obstacle in the general acceptance of laser phototherapy.
As mentioned previously, a typical trick of the Class IV vendor is to make reference to Class 3B papers, with proper documentation of their own products lacking. This was the old trick of LED vendors in the ’90s. The LEDs have, in the meantime, created their own scientific groundwork and do not have to use sales tricks any longer.
You can stop reading here, but if you like, here is the actual text from the book that is supposed to recommend Class IV lasers:
Stronger = better?
The power output of therapeutic lasers has increased radically during the nineties. McKibbin reports that there were about 1800 therapeutic laser units in Canada in 1990. 22% of them were HeNe lasers with an output of 1 mW or less, 35% HeNe lasers with 1-2 mW, 13% 830 nm units with an output up to 5 mW, 3% 830 nm units with an output up to 30 mW, 26% GaAs units with an output of 5 mW or less, and 1% units in the 760-780 range nm with an output up to 30 mW.
Now in 2009, the situation is quite different. HeNe units are being replaced by stronger InGaAlP lasers up to 500 mW, GaAlAs units of 7 000 mW are on the market, and GaAs units of 100 mW and more are available.
Even though it is possible to attain some effects with a 1-2 mW laser, there is no doubt that with a laser 100 times stronger, it is much easier to achieve biostimulating effects, at least if one intends to use treatment periods of the same length. Power density is also very important!
The authors used to have certain misgivings about an “inflation” with respect to the output power of therapeutic lasers. One misgiving was, and still is, the obvious risk of eye damage. The need for protective glasses has previously been exaggerated, but is now becoming more important. Another misgiving is the lack of research in the field of “high-power” therapeutic lasers. So far, insufficient data have been published on these powerful lasers. For the moment, we must rely primarily on our own clinical experience. That experience, however, is so encouraging that it cannot be ignored, even with the lack of scientific support. It would appear that “high-powered” therapeutic lasers will be able to further expand the scope of laser therapy, especially in pain therapy.
The doses previously recommended for laser therapy still hold true, in a way. However, much of what we know about dosage is based upon wound healing studies. This is the field in which both stimulating and inhibiting doses have generally been observed. But a wound is superficial, and the superficial tissue will absorb most of the laser energy. So treating a condition in the inner ear through the bone behind the ear is quite a different matter. The dense bone behind the ear absorbs some 90% of the light energy. Skin and blood absorb another 5%. Thus, 100 J in contact mode means only some 5 J or less in the inner ear. For pain and inflammation in large joints, such as the knee, quite a few joules may be required on the surface before the actual target receives the energy needed.
Using the same amount of energy but with different energy densities will not necessarily trigger the same biological response. Kim [545] used 1.2 J in plastic and aesthetic surgery. The energy was delivered either by a 1000 mW or a 60 mW 830 nm laser (1000 mW × 1.2 sec or 60 mW × 200 sec). Both were effective, but the 60 mW laser was more effective in the initial period of wound healing, while the 1000 mW laser was more effective in the late period.
Are strong lasers better than weaker ones?
YES and NO. Output power should not be too low for its purpose. If the power is too low, it causes unnecessarily long treatment time in order to achieve the required total dose (see more about the dose in the next chapter). Also, if output power is too low, it could result in the power density being too low which is an important parameter in treatment. Nor should output power be too high for its purpose. If the power is too high, the light could burn tanned, coloured skin, tattoos or skin with dark hair. Furthermore, in most countries, there is a power limit of 500 mW (= 0.5 watt), above which the laser may be a Class 4 laser. If so, it usually means that it requires oversight by an MD or DDS, more safety measures, and significantly more regulatory control. Also, if the power is too high, it can result in unintentionally high doses which can give less good treatment results than necessary (see the Arndt-Schulz curve in the next chapter). And finally, time is also an important treatment parameter. Administering a certain number of joules over a certain area using a certain laser power during a certain time, may not give the same result as using a ten times stronger laser during one tenth of the time with unchanged optical configuration. Another way to say this is that the rule of reciprocity is not valid. Some laser companies claim that a Class 4 laser ‘by default’ is better than a Class 3B laser (4 is higher than 3, so it has to be better… right?). This is simply not true. The classification of lasers is a measure of eye hazard, nothing else. While defocused Class 4 lasers may well be used successfully in laser therapy, this does not have anything to do with the laser classification.
Original Source: http://www.laserannals.com/2014/03/22/no-cure-from-litecure/
Efficacy of low level laser therapy and intramuscular electrical stimulation on myofascial pain syndrome.
Sumen A, Sarsan A, Alkan H, Yildiz N, Ardic F. - J Back Musculoskelet Rehabil. 2015;28(1):153-8. (Publication) 379Abstract: PMID: 25061034 [PubMed - in process] Share on Facebook Share on Twitter Share on Google+
Methods: We aimed to evaluate the efficacy of intramuscular electrical stimulation therapy (IMS) and low-level-lasertherapy (LLLT) in patients with MPS.
Results: Patients were randomly divided into three groups. First group were treated with LLLT and stretching exercise. Second group were treated with IMS and stretching exercise. Third group were treated with only stretching exercise. The patients were evaluated through the pain intensity, pain threshold, cervical joint movement range and the neck disability index parameters.
Conclusions: An improvement was found in all parameters for all groups, except for the pain threshold within the control group at the end of the treatment and one month after the treatment. It was found that pain score was significantly lower in Group 1 and 2 at one month after the treatment compared to Group 3. Similarly, it was found that pain threshold score was significantly higher in Group 2 at one month after the treatment compared to Group 3.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25061034
100 positive double blind studies - enough or too little?
Jan Tunér DDS and Lars Hode - (Publication) 4398This published editorial directs people to their book that details many of the positive double blind studies.
Low Level Laser Therapy still has many critics and is not readily accepted as a natural treatment modality in all countries. One main point emphasized by the critics is the lack of scientific documentation. While this was a valid point in the 80s and partly in the beginning of the 90s, is it still a solid argument? There are more than 2000 published studies and the vast majority of these report positive biological effects from Low Level Laser Therapy (LLLT).
The heart of a scientific documentation is the double blind clinical studies. There are some 140 such studies in the field of LLLT and it may come as a suprise to many critics that more than 100 of these are positive. In fact, even most advocaters of LLLT are unaware of this fact. The aim of this Editorial is to disseminate this information to the LLLT community.
Some of the negative double blind studies are well designed and should be taken seriously. Certainly all indications and all parameters cannot work. However, a number of the often quoted negative double blind studies suffer from flaws of several kinds. Some of this is outlined on http://www.laser.nu/lllt/LLLT_critic_on_critics.htm which is a chapter from our recent book "Low Level Laser Therapy - clinical practice and scientific background"
A closer analysis of 100 positive double blind studies will be presented at Laser Florence '99 (October 28-31) and will also appear in the EMLA Millennium laser book.
A weakness in the list is that many double blind studies have only been identified in the abstract form. They may have been published in full at a later stage, but not found by us. 14 studies have only been found as references in reference lists and these have not been found in spite of intensive efforts. For a complete analysis of the 100 positive double blind studies we need the assistance of the visitors of LaserWorld. In the following list abstracts are marked in red and studies not found marked in green. If you have any information about the green studies please contact us. And if you know that an abstract has been published in a journal, please do likewise. The more complete the list is, the better for the LLLT community.
The studies published in journals are listed in full in the book mentioned above.
Atsumi K et al. Biostimulation effect of low-power energy diode laser for pain relief. Lasers Surg Med. 1987; 7: 77.
Barabas K et al. Controlled clinical and experimental examinations on rheumatoid arthritis patients and synovial membranes performed with neodym phosphate glas laser irradiation. Proc. 7th Congr Internat Soc for Laser Surg and Med, Munich June 1987. Abstract no 216a.
Boerner E et al. Double-blind study on the efficacy of the lasertherapy. SPIE Proc. 1996. Vol. 2929: 75-79.
Cheng R. Combined treatments of electrotherapy plus soft laser therapy has synergistic effect in pain relief and disease healing. Surgical and Medical Lasers. 1990; 3 (3): 135
Cieslar G et al. Effect of low-power laser radiation in the treatment of the motional system overloading syndromes. SPIE Proc. Vol 3198. 1997, pp. 76-82.
Emmanoulidis O et al. CW IR low-power laser application significantly accelerates chronic pain relief rehabilitation of professional athletes. A double blind study. Lasers Surg Med. 1986; 6: 173.
Haruki E, Yamaguchi S. Double blind evaluation of low energy laser treatment for painful disease. J Phys Med. 1995; 6: 60-67. (In Japanese with English abstract)
Hopkins G O et al. Double blind cross over study of laser versus placebo in the treatment of tennis elbow. Proc Internat Congr on Lasers, "Laser Bologna". 1985: 210. Monduzzi Editore S.p.A., Bologna. Hoshino H et al. The effect of low reactive level laser therapy in the field of orthopedic surgery. Chronic Pain. 1994; 13: 101-109. (In Japanese with English abstract)
Hoteya K et al. Effects of a 1 W GaAlAs diode laser in the field of orthopedics. In: Meeting Report: The first Congress of the International Association for Laser and Sports Medicine. Tokyo, 1997. Laser Therapy 1997; 9 (4): 185.
Kamikawa K et al. Double blind experiences with mid-Lasers in Japan. 1985. Proc Int Congr on Lasers, "Laser Bologna". 1985: 165-169. Monduzzi Editore S.p.A., Bologna.
Kim J W, Lee J O. Double blind cross-over clinical study of 830 nm diode laser and 5 years clinical experience of biostimulation in plastic & aesthetic surgery in Asians. Lasers Surg Med. 1998; Suppl. 10: 59.
Kinoshita F et al. Clinical evaluation of low-energy, semi-conductor laser therapy in oral surgery - a double blind study. Josai Shika Daigaku Kiyo (Bulletin of Josai Dental University). 1986; 15 (3): 735-742. (in Japanese with English abstract)
Kosaka R et al. Double blind study of low energy diode laser irradiation for chronic pain disorders. J Phys Med. 1993; 4: 156-160.
Kouno A et al. The evaluation of pain therapy with low powerlaser- Comparative study of thermography and double blind test. Biomedical Thermology. 1993; 13: 102-107.
Lonauer G: Controlled double blind study on the efficacy of HeNe-laser beams versus HeNe- plus Infrared-laser beams in the therapy of activated osteoarthritis of finger joints. Clin Experim Rheuma. 1987; 5 (suppl 2) : 39
Lucas C et al. Low level laser therapy bij decubitus statium III. Rapport Hoegschool van Amsterdam. 1994.
Mach E S et al. Helium-Neon (Red Light) Therapy of Arthritis. Rhevmatologia, 1983; 3: 36. (In Russian)
Mester A: Biostimulative effect in wound healing by continous wave 820 nm laser diode. Double-blind randomized cross-over study. Lasers in Med Science, abstract issue July 1988, No 289.
Miyagi K. Double-blind comparative study of the effect of low-energy laser irradiation to rheumatoid arthritis. In: Current awareness of Excerpts Medica. Amsterdam. Elsevier Science Publishers BV. 1989; 25: 315.
Mokhtar B et al. A double blind placebo controlled investigation of the hypoalgesic effects of low intensity laser irradiation of the cervical roots using experimental ischaemic pain. Proc. Second Meeting of the International Laser Therapy Assn., "London Laser", Sept 1992, p 61. Mokhtar B et al. The possible significance of pulse repetition rate in lasermediated analgesia: A double blind placebo controlled investigation using experimental ischaemic pain. Proc. Second Meeting of the International Laser Therapy Assn, "London Laser" Sept 1992. p 62
Neuman I et al. Low energy phototherapy in allergic rhinitis and nasal polyposis. Laser Therapy. 1996. 1: 37.
Palmgren N et al. Low Level Laser Therapy of infected abdominal wounds after surgery. Lasers Surg Med. 1991; Suppl 3:11.
Poliakova A G., Gladkova N D, Triphonova T.D. Laserpuncture in patients with rheumatoids arthritis. Abstracts of ICMART '97 International Medical Acupuncture Symposium, Nicosia, Cyrprus, March 26-29 1997.
Rochkind S et al. Double-blind Randomized Study Using Neurotube and Laser Therapy in the Treatment of Complete Sciatic Nerve Injury of Rats. Proc. 2nd Congr World Assoc. for Laser Therapy, Kansas City, 1998.
Roumeliotis D et al. 820nm 15mW 4J/cm2, laser diode application in sports injuries. A double blind study. Proc. Fifth Annual Congress British Medical Laser Ass. 1987.
Ryo E et al. Double blind test of low energy laser radiation treatment. Evaluation of effectiveness for shoulder stiffness, arthralgia etc. Pain Clinic. 1986; 7: 185-192. (In Japanese with English abstract)
Saeki N et al. Double blind test for biostimulation effects on pain releif by diode laser. 1989. Laser Surgery; 1066: 93-100.
Sasaki K et al. A double-blind controlled study on free amino acid analysis in CO2 laser burn wounds in the mouse model following doses of low incident infrared (830 nm) diode laser energy. Proc. 2nd Meeting if the Internat Laser Therapy Assn., London, 1992, p.4.
Sato K et al. A double blind assessment of low power laser therapy in the treatment of postherpetic neuralgia. Surgical and Medical Lasers. 1990; 3 (3): 134.
Scudds R A et al. A double-blind crossover study of the effects of low-power gallium arsenide laser on the symptoms of fibrositis. Physiotherapy Canada.1989; 41: (suppl 3): 2.
Taghawinejag M et al. Laser-Therapie in der Behandlung kleiner Gelenke bei chronischer Polyarthritis. Z Phys Med Baln Med Klin. 1985; 14.
Tsurko V V et al. Laser therapy of rheumatoid arthritis. A clinical and morphological study. Ter Arkh. 1983; 55 (7) 97-102. (Russian).
Umegaki S et al. Effectiveness of low-power laser therapy on low back pain. Double blind comparative study to evaluate the analgesic effect of low power laser therapy on low-back pain. The Clinical Report. 1989; 23: 2839-2846. (In Japanese with English abstract)
Vélez-Gonzalez M et al. Treatment of relapse in herpes simplex on labial and facial areas and of primary herpes simplex on genital areas and "area pudenda" with low power HeNe-laser or Acyclovir administred orally. SPIE Proc. 1995; Vol. 2630: 43-50
Willner R et al. Low power infrared laser biostimulation of chronic osteoarthritis in hand. Lasers Surg Med. 1985; 5: 149.
Wylie L et al. The hypoalgesic effects of low intensity infrared laser therapy upon mechanical pain threshold. Lasers Surg Med. 1995; Suppl 7: 9.
Yamaguchi M et al. Clinical study on the treatment of hypersensitive dentine by GaAlAs laser diode using the double blind test. Aichi Gakuin Daigaku Shigakkai Shi - Aichi-Gakuin Journal of Dental Science. 1990; 28( 2): 703-707. (in Japanese)
Yoh K et al. A clinical trial for treatment of chronic pain in orthopedic diseases by using 150 mW diode laser system. Result of double blind test. Chronic Pain; 13: 96-100.(In Japanese with English abstract)
Original Source: http://www.laser.nu/lllt/lllt_editorial3.htm
Quantitative In Vivo Imaging of Tissue Absorption, Scattering, and Hemoglobin Concentration in Rat Cortex Using Spatially Modulated Structured Light
David J. Cuccia, David Abookasis, Ron D. Frostig, and Bruce J. Tromberg. - 2009 (Book) 450212.1. INTRODUCTION
Significant changes in blood flow or in the integrity of cerebral vessels are believed to cause cerebrovascular disease (CVD) and to contribute to dementias including Alzheimer’s disease [1]. Stroke, the most serious form of CVD, is one of the leading causes of death and adult disability worldwide. Acute treatments for stroke, however, are severely limited. Neuroprotective drugs under development show promise at halting the ischemic cascade, but as yet, no such compound has received federal approval in the United States. One of the biggest limitations to this development is the lack of understanding of the mechanisms by which cerebral vessels react to factors such as ischemia, inflammation, blood pressure changes, metabolic demands, and trauma [2]. In order to address these fundamental questions, functional brain imaging techniques such as fMRI and intrinsic signal optical imaging (ISOI) have emerged as tools to visualize and quantify cerebral hemodynamics.
In the neuroscience community, ISOI has long been used to study the organization and functional architecture of different cortical regions in animals and humans [3–5] (see other chapters in this book). Three sources of ISOI signals that affect the intensity of diffusely reflected light derive from characteristic physiologic changes in the cortex. For functional neuronal activation, these have been observed to occur over a range of timescales, including (1) light scattering changes, both fast (over 10 s of milliseconds) and slow (i.e., > ~0.5 s) (2) early (~0.5–2.5 s) absorption changes from alterations in chromophore redox status, i.e., the oxy/deoxy-hemoglobin ratio (known as the “initial dip” period), and (3), slower (~2–10 s) absorption changes due to blood volume increase (correlated with the fMRI BOLD signal). Light scattering changes have been attributed to interstitial volume changes resulting from cellular swelling, organelle swelling due to ion and water movement, capillary expansion, and neurotransmitter release [6,7]. The slower absorption factors have been demonstrated to correlate with the changes in metabolic demand and subsequent hemodynamic cascades following neuronal activation [4,8,9].
Using animal models of acute and chronic brain injury, ISOI has been used to quantify the acute hemodynamic events in response to stroke, including focal ischemia and cortical spreading depression (CSD) [10–21]. Researchers have also used ISOI to locate and quantify the spatial extent of the stroke injury, including ischemic core, penumbra, and healthy tissue zones [18,22]. CSD also plays a key role in migraine headache, and recent laser speckle imaging studies have revealed the neurovascular coupling mechanism to the transmission of headache pain [23,24].
To fully understand the underlying mechanisms in vascular changes associated with cerebrovascular diseases such as stroke, an optical imaging technique that has the capability to rapidly separate absorption from scattering effects can enhance the information content of traditional ISOI, enabling (1) more accurate quantitation of hemodynamic function, (2) isolation of the electro-chemical changes characterized by light scattering, and (3) longitudinal chronic injury studies of function where structural reorganization due to neovascularization can cause significant alterations in scattering [25,26].
Quantitative diffuse optical methods [27] such as spatially-resolved reflectance, diffuse optical spectroscopy (DOS), and tomography (DOT), and diffuse correlation spectroscopy (DCS) possess exquisite sensitivity to these functional and structural alterations associated with brain injury, and have been applied to the study of CSD [11,15,28]. DOS and DOT utilize the near-infrared spectral region (600–1000 nm) to separate and quantify the multispectral absorption (μa) and reduced scattering coefficients (μs′), providing quantitative determination of several important biological chromophores such as deoxy-hemoglobin (HbR), oxy-hemoglobin (HbO2), water (H2O), and lipids. Concentrations of these chromophores represent the direct metrics of tissue function such as blood volume fraction, tissue oxygenation, and edema. Additionally, the scattering coefficient contains important structural information about the size and density of scatterers and can be used to assess tissue composition (exctracellular matrix proteins, cell nuclei, mitochondria) as well as follow the process of tissue remodeling (wound healing, cancer progression). DOS utilizes a limited number of source-detector positions, e.g., 1–2, but often employs broadband content in temporal and spectral domains [29]. In contrast, DOT typically utilizes a limited number of optical wavelengths (e.g., 2–6) and a narrow temporal bandwidth, but forms higher resolution images of subsurface structures by sampling a large number of source-detector “views.” To achieve maximal spatial resolution, the ideal DOT design would employ thousands of source-detector pairs and wavelengths. However, several engineering considerations including measurement time and instrument complexity currently limit the practicality of this approach.
In this chapter we present the basic principles of a new, noncontact quantitative optical imaging technology, modulated imaging (MI) [30–32], and provide examples of MI performance in 2 rat models of brain injury, cortical spreading depression (CSD) and stroke. MI enables both DOS and DOT concepts with high spatial (<1 mm) and temporal resolution (<1 s) in a simple, scan-free platform. MI is capable of both separating and spatially-resolving optical absorption and scattering parameters, allowing wide-field quantitative mapping of tissue optical properties. While compatible with time-modulation methods, MI alternatively uses spatially modulated illumination for imaging of tissue constituents. Periodic illumination patterns of various spatial frequencies are projected over a large area of a sample. The diffusely reflected image is modified from the illumination pattern due to the turbidity of the sample. Typically, sine-wave illumination patterns are used. The demodulation of these spatially modulated waves characterizes the modulation transfer function (MTF) of the material, and embodies the sample optical property information.
12.2. METHODS AND INSTRUMENTATION
12.2.1. Modulated Imaging Spectroscopy
The MI instrument platform was introduced originally by Cuccia et al. [30] Based on this design, we have developed a custom multispectral near-infrared (NIR) MI spectroscopy system capable of imaging between 650 and 1000 nm. A diagram of this system is shown in Figure 12.1.

FIGURE 12.1
Modulated imaging platform. QTH—quartz tungsten halogen; L1—aspheric condenser; H—hybrid hot mirror; DMD—digital micromirror device; L2—projection lens; L3—camera lens; LCTF—liquid crystal tunable (more...)
Broadband NIR illumination is provided by an intensity-stabilized 250 W quartz-tungsten-halogen (QTH) lamp (Oriel QTH Source with Light Intensity Controller, Newport Corporation-Oriel Instruments, Stratford, Connecticut). Light is collimated and refocused with a pair of aspheric F/#0.7 optical lens systems (Oriel Aspherab). A custom-sized 3.5 in square hybrid hot mirror (Reynard Corporation, i.e., R00670-00) was placed between the lenses to limit the illumination to wavelengths below 1000 nm. Light engine optics taken from a digital projector (NEC HT1000) serve to homogenize and direct the light onto a 0.7 in digital micromirror device (DMD Discovery™ 1100 with ALP Accessory Package, ViALUX, Germany). Grayscale spatial sinusoid patterns are projected at 400 Hz using the ViALUX software development toolkit, which generates the necessary pulse-width modulation of binary sub-frames to produce a specified grayscale bit-depth (1–8 bits). Finally, a fixed focal length (f = 100 mm) projection lens illuminates the tissue at a slight angle from normal with a 15 × 25 mm illumination field. Detection was performed at normal incidence using a CRI Nuance™ camera system, which combines a 12-bit CCD camera and a liquid crystal tunable filter (LCTF; λ = 650–1100 nm, Δλ = 10 nm). To avoid specular reflection, crossed linear polarizers are used in the illumination and detection arms. For this system, the former is a 1.5 in diameter NIR linear polarizer (Meadowlark Optics, VLM-200-IR-R) placed immediately after the projection lens, and the first stage of the Nuance LCTF serves as the latter. The DMD, CCD, and LCTF are controlled via USB by a laptop computer, and synchronized using LabVIEW software (LabVIEW 8, National Instruments), enabling fast acquisition of a series of patterns with various spatial frequencies.
12.2.2. SFD Measurement, Calibration, and Modeling
A detailed description of SFD measurement, calibration, and diffusion modeling is provided by Cuccia [32]. In this work, we modeled diffuse reflectance using a transport-based White Monte Carlo (WMC) method [33,34]. Previously, we have found that compared with Monte Carlo, (1) diffusion predictions over- and underestimate low- and high-frequency diffuse reflectance, respectively, and (2) the quantitative accuracy of diffusion degrades with decreasing albedo [32]. Due to the moderate albedo of brain tissue (μs′/μa ~ 10–20), we chose to analyze all brain data with the WMC approach. This homogeneous tissue model is a significant simplification of the multilayered rat brain, and more work is necessary to accurately model this complex system. We discuss further the consequences of our simple model in Section 12.2.5.
12.2.3. Optical Property Inversion Methods
In this chapter, we use two inversion methods to calculate the absorption and reduced scattering from measurements of diffuse reflectance. When high measurement precision is desired, we use a “sweep” in spatial frequency space, producing an overdetermined set of diffuse reflectance measurements, which can be fitted to our WMC forward model predictions using least-squares minimization. This method is performed for all spatially averaged region analysis of optical properties and chromophores. When increased acquisition and/or processing speed is desired, we alternatively use a rapid two-frequency lookup table method based on cubic spline interpolation [32]. This data can be achieved with a minimal 3-phase, single frequency image set (by demodulating and averaging the images to obtain AC and DC amplitude maps, respectively). On typical personal computers this approach is capable of millions of inverse lookup calculations per second, and is therefore used to calculate all high-resolution images including time sequences. The signal-to-noise ratio (and thus the measurement precision) of either approach is limited by the data sampling, with the two-frequency method having a lower precision with the tradeoff of higher acquisition and processing speed.
12.2.4. Spectral Analysis-Chromophore Calculation
The quantitative absorption coefficient is assumed to be a linear (Beer’s law) summation of individual chromophore absorption contributions:
where ci and ?i(λ) represent chromophore concentrations and molar extinction coefficients, respectively. Using reported extinction coefficients of HbO2/HbR35 and H2O,36 we can invert Equation 12.1 and calculate tissue chromophore concentration separately at each pixel by linear least-squares fitting to the multispectral absorption images. Total hemoglobin (HbT) and oxygen saturation (StO2) can then be calculated as HbT = HbR + HbO2 and StO2= HbO 2/(HbR + HbO2) * 100, respectively.
12.2.5. Optical Property Mapping: Resolution Versus Quantitation
On a pixel-by-pixel basis, diffuse reflectance versus spatial frequency is fitted to the WMC forward model to extract the local absorption and reduced scattering optical property contrast. This process is repeated for each wavelength, resulting in multi-spectral absorption and scattering spectra at each pixel. The measured contrast from discrete absorbers and scatterers on millimeter and submillimeter spatial scales, however, will possess partial volume effects in all three spatial dimensions. This is due to the physical light transport length scales in tissue, limiting the true x-y resolution of optical property contrast to many detector pixels [32]. This phenomenon is not unique to MI, but present in all planar reflectance imaging measurements of turbid media. Absorption and scattering are calculated using a homogeneous reflectance model, extracting a locally averaged sampling of optical property contrast. Based on simulations of the tissue MTF for varying optical properties [32], we expect the resulting image resolution to scale directly with the transport length, l* = (μa + μs′)− 1, and the spatial frequency of illumination. In this chapter, we place quantitative emphasis on average optical properties and chromophores measured over a field of view that is greater than l*. Spatial maps and videos of these parameters are displayed and referred to as “contrast maps,” with the caveat that high resolution features will exhibit degraded quantitative accuracy.
12.2.6. In Vivo Rat CSD Experiments
12.2.6.1. Animal Preparation
MI spectroscopy measurements were performed on an in vivo Wistar rat model with a thinned-skull preparation. All procedures were performed in accordance with approved IACUC protocol guidelines. The animals were anesthetized, placed in a stereotaxic frame, their skulls thinned and glass coverslip applied. This preparation is described in detail by Masino et al. [37] The resulting thinned skulls allowed direct imaging of the cortex over a 5 × 7 mm field-of-view (whisker barrel cortex, centered at the C2 location). In order to investigate the sensitivity of MI toward studying acute cortical injury, we induced cortical spreading depression (CSD) by applying 1 M KCl solution to the surface of the cortex through a perforated section of skull and dura, located approximately 3 mm above the camera’s imaging field.
12.2.6.2. MI Measurement Protocol
For each of three animals, our MI measurement protocol was twofold. Prior to CSD induction, baseline spatial modulation data were acquired at 6 spatial frequencies (3-phase projections each) from 0 to 0.26 mm−1, at 10 nm intervals over the entire range between 650 and 980 nm. Depending on the wavelength, image acquisition times ranged from 200 ms to 4 s, with total spectral imaging time of approximately 30 s per spatial pattern. The entire measurement (34 wavelengths, 3 phases, 6 frequencies) was repeated three times for statistical averaging yielding an entire measurement time of approximately 30 min.
Next, rapid dynamic measurements were performed, beginning 1 min prior to K+Cl− administration. Here, a significantly reduced data set was chosen in order to achieve high temporal resolution. Two spatial frequencies (0 and 0.26 mm−1) were acquired with three phase projection images, as described in Section 12.2.2, at each of four wavelengths (680, 730, 780, and 830 nm). The resulting 12 images took in total 6 s, permitting a repetition rate of 10 measurements per minute. The animals were followed for a period of 10 min for rats 1 and 2, and a period of 30 min for rat 3.
All images in this study were smoothed by 2D convolution with a Gaussian filter function (FWHM = 3 pixels), and baseline repetitions were averaged prior to data processing. Additionally, time-series data were post-processed by smoothing slightly in time (Gaussian FWHM of 2 timepoints = 12 s).
12.2.6.3. Spatial Frequency Sensitivity Analysis
Because of the differential absorption sensitivity at low and high frequencies, optimal optical property separation is achieved when a large range of frequencies is used [31]. In Figure 12.2a, we depict this differential sensitivity using diffuse reflectance (MTF) predictions versus frequency, increasing μa by 100% from 0.02 (black line) to 0.04mm−1 (gray line). This is done for two values of μs′, 0.6 (solid lines) to 1.2mm−1 (dashed lines), simulating a 100% change in scattering. Notice that the low frequencies have a significant reflectance change due to absorption, while high frequency reflectance remains nearly unchanged. Conversely, reflectance changes due to scattering are observed at all spatial frequencies. In Figure 12.2b, we further visualize this by plotting the reflectance sensitivity to 1% changes in absorption and scattering. Whereas DC reflectance is equivalently sensitive to a fractional change in either absorption or scattering, at high spatial frequencies absorption contrast is lost while scattering contrast is retained. For instance, notice that at our maximum measurement frequency of 0.26 mm−1 the reflectance is roughly 24 times more sensitive to scattering compared to absorption (ΔRd = 0.56 μs′ versus 0.024 * 10−3 for μa). This plays an important role in Section 12.3.2 during our discussion of dynamic scattering measurement.

FIGURE 12.2
(a) Reflectance contrast in absorption and scattering covering a typical range of brain optical properties. (b) The frequency-dependent sensitivity to absorption (black line) and scattering (gray line), respectively. Reflectance at fx = 0.26 mm−1 (more...)
In realistic heterogeneous tissues, a tradeoff exists between maximizing the frequency range for optical property accuracy and obtaining similar sampling volumes. As tissue is a low-pass spatial filter, high frequencies are attenuated quickly with depth. Using diffusion-based forward modeling, we have estimated mean sampling depths at 650 nm using measured average background optical properties of brain tissue. This was done by predicting the depth sensitivity to contrast from a planar perturbation in absorption, given a background fluence profile from spatial frequencies 0 and 0.26 mm−1. Based on these results, we observe qualitatively similar depth sampling, with mean depth sampling ranging between 2.5 mm and 1.2 mm (for fx = 0 and 0.26 mm−1, respectively). In all cases maximal sensitivity was found in the first 1–2 mm, where cortical hemodynamic changes occur.
12.3. RESULTS AND DISCUSSION
12.3.1. Baseline MI Spectroscopy
In Figure 12.3a we show a grayscale planar reflectance image of the cortical region of rat 1 at 650 nm. A dotted-line box denotes the region-of-interest (ROI) used for analysis, selected for its uniform illumination and the absence of cerebral bruising. The Monte Carlo-model fitting of spatial frequency data allows calculation of the absorption and reduced scattering coefficients. In Figure 12.3b we show the spatially averaged diffuse reflectance at 650 nm and the corresponding multi-frequency fit. Excellent agreement is observed between measurement data and the model-based fit, with derived μa and μs′ coefficients of 0.033 and 0.70 mm−1, respectively.

FIGURE 12.3
(a) Reflectance map for rat 1, showing the 3.8 × 5.9 mm region chosen for quantitative analysis. (b) Sample MTF reflectance data (squares) and fit (solid line) at 650 nm. (c) Recovered optical property maps (above) and corresponding image histogram (more...)
Analysis of multifrequency reflectance data separately at each pixel results in spatial maps of absorption and reduced scattering contrast. In Figure 12.3c, we plot the μa and μs′ maps recovered at 650 nm for rat 1. Note the strong absorption in the vein region, due to a large absorption by HbR at this wavelength. Below the images, we show histogram distributions of the corresponding quantitative maps above, indicating the degree of spatial variation in recovered optical properties. The mean and standard deviation for the pixel-wise μa and μs ′ were 0.030 ± 0.007 mm−1 and 0.63 ± 0.13 mm−1, respectively. These statistical results are in good agreement with the spatially averaged reflectance fit from Figure 12.3b, suggesting that our simple pixel-wise fitting approach yields optical properties similar to that calculated using a global analysis.
By mapping the absorption coefficient at multiple wavelengths, we can perform quantitative spectral imaging of tissue. In Figure 12.4, we summarize the baseline spectroscopy results for all three animals. In Figure 12.4a we show the μa (left) and μs′ (right) coefficients versus wavelength (circles) recovered from spatially averaged fitting. Data for rat 1 is shown in black (rat 2 in dark gray; rat 3 in light gray). Note the distinct spectral features in absorption, resulting from oxy- and deoxy-hemoglobin (HbO2, HbR), and water (H2O) absorption. The calculated scattering coefficient generally decays with increasing wavelength, and the results from a power law (μs ′ = A·λ(nm) −b, solid lines) fit are shown. A small residual coupling is observed between measured scattering and absorption spectral features. In particular, the scattering at the shortest and longest wavelengths appears to be underestimated by 5–10%, occurring where the corresponding absorption is highest (due to HbR and H2O, absorption features, respectively). Based on our experiments in layered tissue phantoms [38], we believe this effect is primarily due to frequency-dependent probing volumes in the presence of depth-heterogeneous structures.

FIGURE 12.4
(a) Average μa (left) and μs′(right) spectra over entire ROI (circles). HbO 2, HbR, and H2O concentrations are determined by subsequent least-squares fitting (solid lines) of molar extinction coefficients to the absorption. Data (more...)
Simultaneous linear fitting of the absorption to known extinction coefficients yields measures of chromophore concentration. Shown in Figure 12.4a, multispectral fitting (solid line) for rat 1 yields HbO2, HbR, H2O, HbT and StO2 values of 56.3 μM, 33.2 μM, 63.9%, 89.6 μM, and 56.3%, respectively. Tabulated results of chromophore values for all three animals are shown in Figure 12.4b. Lipid absorption near 930 nm was not apparent in the μa spectrum, and when included in the spectral analysis was not found to significantly affect the results. The small absorption “bump” at 900–910 nm is an artifact of imperfect phantom calibration due to the presence of a sharp, strong silicone absorption peak that is present in the phantom.
We note that the solution for chromophore concentration is well-determined when the number of wavelengths is at least equal to the number of chromophores. Therefore, as few as two wavelengths can be used to separate HbO2 and HbR (if a constant value of H2O is assumed). Repeating the above analysis with 780 and 830 nm only (assuming H2O = 65%) yields results for HbO2 and HbR within 10% of those from full spectral fitting. Repeating the above analyses using a simple diffusion-based model provided qualitatively similar results for absorption and scattering spectra, but in general was found to overestimate the absorption coefficient by 10–25%.
Absorption spectra at each pixel can be separately analyzed to yield spatial maps of local HbO2, HbR, and H2O distribution, shown in Figure 12.5. Notice the high concentration of HbR over the large superficial draining vessel (venous) regions, also reflected in the StO2 image, highlighting the effect of tissue oxygen extraction. Conversely, notice that the high albedo regions with less structural detail are highly oxygenated, with StO2 levels between 60 and 70%. Lastly, the H2O map reveals a relatively homogeneous distribution of water.

FIGURE 12.5
Chromophore fits to absorption spectra at each pixel yield maps of local HbO2, HbR, and H2O concentration (left). Total hemoglobin (HbT) and oxygen saturation (StO2) maps can then be calculated from HbO2 and HbR.
12.3.2. Dynamic MI Spectroscopy of CSD
We performed measurements of CSD in each of the three rats, as described in Section 12.2.3. The results are presented as follows. We first present data for a single animal, choosing rat 3 for its long observation period of 30 minutes. Three ROIs are selected for analysis, and baseline MI spectroscopy results are reported for each of these regions. Next, the observed dynamic time courses of diffuse reflectance, optical properties, and chromophore concentrations are shown for each ROI. We then present the full spatio-temporal dynamic contrast data for rat 3 (2D + time) in the form of “snapshot” images.
Figure 12.6 summarizes the baseline spectroscopy measurements for rat 3. In Figure 12.6a, we show three regions of interest superimposed on the DC reflectance map, chosen to highlight three different characteristic temporal profiles observed within the field of view. In Figure 12.6b we show the baseline spectral fits for each of these regions, and in Figure 12.6c we tabulate the resulting calculated chromophore concentrations. In general, Region A (black) is a high albedo region lacking any large blood vessels, whereas Regions B (dark gray) and C (light gray) include high-absorption blood vessels and mild cerebral bruising from surgery. These differences are apparent in their recovered absorption spectra and fits, with on average 27% higher HbT, and 32% lower saturation in the vascular regions. Also, 7% higher H2O is found in Regions B and C, which may indicate increased edema due to bruising.

FIGURE 12.6
Regionwise spectral analysis of rat 1 baseline data including the respective (A) ROIs, (B) spectral absorption data (circles) and fit (lines), and (C) tabulated recovered chromophore data for each region
In Figures 12.7–12.9 (for regions A–C, respectively), we present the temporal dynamics of CSD in each ROI of rat 3 as measured by MI. In part (a) of each figure, we plot the multispectral diffuse reflectance changes at fx = 0 mm−1 (DC, top) and fx = 0.26 mm−1 (AC, bottom). In part (b), we plot the recovered Δμa (top) and Δμs′ (bottom) optical properties at each wavelength. While absolute values of diffuse reflectance and optical properties are measured separately at each time point, for visualization purposes all data are displayed as a change from that prior to KCl administration. Absolute optical property values at t = 0 (not shown) demonstrate excellent agreement (~5–10%) with full multifrequency baseline data.

FIGURE 12.7
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx =.26 mm−1, bottom) for Region A of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) coefficients. (more...)

FIGURE 12.8
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx = 0.26 mm−1, bottom) for Region B of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) (more...)

FIGURE 12.9
(A) Multispectral diffuse reflectance at DC (fx = 0 mm−1, top) and DC (fx = 0.26 mm−1, bottom) for Region C of rat 3 over approximately 30 min. (B) Corresponding recovered multispectral absorption (top) and reduced scattering (bottom) (more...)
Looking first at the reflectance time courses of Figure 12.7a (Region A), we see in general a series of three CSD events over the 30 minutes, with each transient event occurring for approximately 4.3 minutes. The first event occurs at minute 2.9 after KCl application, indicating an initial latency between the insult and the first resulting spreading depression wave. Reflectance contrast is present in both DC and AC frequency components, but with markedly different signatures. Generally, the DC time course shows a slow, gradual decay, punctuated by sharp, wavelength-dependent spikes/dips (for short/long wavelengths, respectively). Alternatively, the AC signature contains three sets of transient dips consistent across all wavelengths, with final values leveling off progressively lower than baseline. Discussed in detail in the following paragraph, we believe these AC changes are due primarily a result of optical scattering and may be related to neuronal depolarization. The corresponding derived optical properties in Figure 12.7b reflects this, with μs′ trends tracking directly with the measured AC reflectance. As expected, μa trends reveal similar wavelength-dependence of the DC reflectance (with opposite polarity), reflecting changes in HbO2 and HbR.
In Section 12.2.3.3 we noted that the diffuse reflectance at fx = 0.26 mm−1 is 23 times more sensitive to scattering changes compared to absorption. In this context, we propose that the observed magnitude of the CSD-induced AC reflectance changes can only be explained by changes in optical scattering. To concretely illustrate this point, we pick as an example the observed 780 nm AC diffuse reflectance dip in Figure 12.7a at t = 3.7 min of -0.003. Here, the corresponding change in reduced scattering in Figure 12.7b, Δμs′, is calculated to be −0.03 mm−1. In order for this change to instead be due to an absorption-only event, μa would need to increase by 121% from baseline (from 0.038 to 0.084 mm−1). This increase would also need to be accompanied by a drop in Rd (fx = 0 mm−1) of 0.12 (33%), whereas the actual observed DC reflectance only drops by 0.008 (<1%) and thus cannot explain the change. Secondly, we note that the three sets of AC reflectance dips occur consistently across all four wavelengths. While an approximate 120% increase in HbT could induce this decrease at high frequency, it would also require a large broad-wavelength decrease in the DC reflectance. We instead observe during these events that the DC increases at short wavelengths while the DC decreases at long wavelengths, suggesting primarily an exchange between HbO2 and HbR volume fractions, as opposed to a dramatic HbT change.
Regions A–C (Figures 12.7–12.9) were chosen to highlight three different time signatures observed in the field of view during the CSD dynamics. The most contrasting feature between all three regions is the measured AC reflectance and the derived scattering coefficient. In Region B (Figure 12.8), each CSD event appears to cause a biphasic scattering change, with a sharp increase and then decrease, whereas a monophasic dip was observed in Region A (Figure 12.7). Region C (Figure 12.9) appears even more complex with a triphasic rise-dip-rise temporal profile. We observe that Regions A to C are located with increasing proximity to the CSD induction point (3 mm above the imaging field).
Because fractional changes in scattering and absorption have an equal (and opposite) effect on DC reflectance (see Section 12.2.3.3), any scattering (i.e., pathlength) changes measured here could be misinterpreted as absorption events with traditional ISOI analyses (i.e., DC reflectance only). In our observations, the measured scattering change of up to −0.05 mm−1 would be interpreted as an increase in absorption of up to +0.005 mm−1, more than the maximum measured absorption change for wavelengths 730, 780, or 830 nm in any of the three regions. In order to account for differential pathlength changes, Kohl et al. proposed a multispectral model [39], which they used to differentiate dynamic scattering and absorption changes using ISOI. This approach improves ISOI accuracy, and has been generally adopted as the method of choice for quantitative functional imaging. For dynamic measurements, we see MI as an improvement over this approach as it alternatively uses frequency domain measurements at a single wavelength to derive absolute scattering and absorption coefficients. This potentially provides a simplified single-wavelength measurement apparatus for detection of scattering, and also avoids potential mis-estimation of background optical properties.
Light scattering changes induced by spreading depression have been reported previously, and a comprehensive review is provided by Somjen. With in vivo spatially resolved reflectance measurements, Kohl et al. [11] separated absorption from scattering and observed a biphasic scattering response similar to that of Region A. With simultaneous laser scattering and electrophysiological measurements, both Jarvis et al. and Tao et al. found a strong correlation between electrical and optical scattering changes [12,13,40]. Tao et al. noted spatial heterogeneity in the dynamic spreading depression (SD) waveform related to the proximity to the SD induction site, similar to our results.
Using linear spectral analysis of absorption at all four wavelengths, we calculated the time-dependent chromophore concentration for Regions A, B, and C, presented in Figure 12.10A,B,C, respectively. In each region, the calculated baseline concentrations of H2O were assumed to be constant. All three regions exhibit remarkably similar trends in HbR, HbO2, HbT, and StO2. This similarity is not clear in the DC traces of Figures 12.7–12.9, further highlighting the benefit of accurate separation of μa and μs′. Focusing on the first CSD event, there is a very consistent signature of: (1) a 2-minute latency post-KCl administration, (2) a 30-second period of decreasing StO2 (3) a dramatic spike in both StO2 (3–10%) and HbT (2–4 μM) with rise and decay times of approximately 1 minute each. For each region, the final StO2 is approximately 5–10% lower than baseline, while the HbT restores to baseline values. This process repeats again twice more, except that the phase (2) desaturation appears to be absent. Additionally, in the “vessel” Region 3, we observe a gradual increase in HbT over the 30 minutes, indicating chronic blood pooling.

FIGURE 12.10
Recovered HbR, HbO2, HbT, and STO2, for ROIs A, B, and C (top, middle, and bottom), recovered by analysis of the multispectral absorption coefficients from Figures 12.7–12.9b (top).
We show in Figure 12.11 the spatio-temporal evolution of both chromophore concentration and scattering changes from the first SD wave in rat 3. These are depicted in the form of a time derivative, i.e., (C(tn + 1) − C(tn))/(tn + 1 − tn), where C represents concentration/saturation/scattering values and tn represents time of acquisition for data point n. This visualization is appealing as it highlights the changes with high contrast [18]. From left to right, we show HbO2, HbR, HbT, StO2, and μs′. Notice the wave in scattering which propagates from top right to bottom left, at a rate of approximately 3 mm/min. An increase, or “spike” in scattering is observed initially in the top right hand corner, in close proximity to the location of KCl administration. Note the large spikes in HbT and StO2 due to vascular activity from depression wave propagation through the measurement field. We observe a transient increase in saturation and blood volume. Over the longer time periods, however, we observe a slow, sustained trend toward hypoxia in the vein regions.

FIGURE 12.11
Spatio-temporal evolution of the hemodynamic and neural scattering response during a single spontaneous CSD event in rat 3. For visualization, a time derivative of the image sequence is displayed to highlight changes.
The spatio-temporal evolution of the scattering coefficient in Figure 12.11 reveals a spatially defined scattering wave (reduction in μs′) that precedes hemodynamic changes. The scattering drop is presumed to be a consequence of neuronal depolarization accompanying CSD. This observed wave pattern has been shown previously with reflectance ISOI and attributed to blood volume changes [18]. Interestingly, the scattering depolarization wave is clearly followed in space and time by the increase in deoxyhemoglobin (HbR), decrease in saturation (StO2), and drop in oxyhemoglobin (HbO2); changes that are consistent with depolarization-induced neural tissue oxygen consumption.
12.3.3. Dynamic MI Spectroscopy of Stroke
In order to assess the sensitivity of MI to stroke, we conducted preliminary studies in a rat middle cerebral artery occlusion (MCAo) model, the most commonly involved artery in ischemic strokes. The left MCA was surgically cauterized using monopolar cautery or ligated to produce a permanent stroke. Figure 12.12 shows pre-versus post-MCAo results for a representative animal. Data were acquired at 5 wavelengt
Original Source: https://www.ncbi.nlm.nih.gov/books/NBK20233/

Abstract: PMID: 26447605 [PubMed - in process] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26447605
Abstract: PMID: 26406994 [PubMed - as supplied by publisher] PMCID: PMC4583288 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26406994
Abstract: PMID: 26394337 [PubMed - in process] Share on Facebook Share on Twitter Share on Google+
Methods: Thirty-two male New Zealand rabbits were randomly divided into four groups of eight animals each, one control group (nonirradiated animals) and three experimental groups that received LLLT (group E5 = 5 J per session; group E10 = 10 J per session; group E20 = 20 J per session). The mandibular left incisor was surgically extracted in all animals, and a nanoparticle-treated-surface osseointegrated implant was placed immediately afterward. The experimental groups were irradiated with aluminum-gallium-arsenide laser diode every 48 hours over a 13-day period for a total of seven sessions. Implant stability quotients (ISQs) were measured at the time of implant placement and 30 days after the last LLLT session. The animals were then euthanized and dissected, and histologic slides of the implant region were obtained for BIC evaluation.
Results: Significant differences in ISQ were detected between groups before and after LLLT, with group E20 showing significantly higher values than controls. The percentage of BIC was also significantly higher in group E20 than in control animals.
Conclusions: Laser therapy at a dose of 20 J per treatment session, based on the irradiation protocol used in this study, was able to significantly increase ISQ values and BIC after implant placement, indicating that laser irradiation effected an improvement in peri-implant bone healing.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26394337
Background: Osteoarthritis (OA) is a highly prevalent and disabling disease. It is estimated that by 2030 the prevalence of symptomatic OA could reach 30 % of the population above 60 years. This randomised controlled trial will investigate the effect of low-level laser therapy (LLLT) and static stretching exercises, as monotherapy and in combination, on pain, quality of life, function, mobility, knee range of motion (KROM) and hamstring shortening in participants with knee OA.
Abstract: Abstract BACKGROUND: Osteoarthritis (OA) is a highly prevalent and disabling disease. It is estimated that by 2030 the prevalence of symptomatic OA could reach 30 % of the population above 60 years. This randomised controlled trial will investigate the effect of low-level laser therapy (LLLT) and static stretching exercises, as monotherapy and in combination, on pain, quality of life, function, mobility, knee range of motion (KROM) and hamstring shortening in participants with knee OA. METHODS: This study will involve 145 people aged 50-75 years with symptomatic-radiographic knee OA. It will consist of two types of treatments: Low-level laser therapy (LLLT) and stretching exercises. The patients will be randomly allocated to five groups LLLTACTIVE+Stretch, LLLTPLACEBO+Stretch, Stretch, LLLT and Control (n = 29 each). Treatment frequency will be three sessions/week for all active groups. LLLT will involve the use of a Gallium-Arsenide laser (904 nm, 40 milliwatts, 3 J/point, 27 J/knee) over 24 sessions for the monotherapy group and 9 sessions for the LLLT+Stretch groups. Stretching will consist of seven exercises completed over 24 sessions. The control group will receive a booklet. Participants will be treated for 2 months (Stretch, LLLT and Control groups) or 3 months (LLLT + Stretch groups). Participants and the outcome assessor will be blind to treatment allocation throughout the study. The primary outcome is pain measured by Visual Analogue Scale. Secondary outcomes include quality of life assessed by Western Ontario and McMaster Universities Arthritis Index, function by Lequesne Algofunctional Index, mobility by Timed Up and Go Test, KROM by goniometry of knee flexion and hamstring shortening by popliteal angle. The statistical method will follow the principles of per-protocol analysis. DISCUSSION: Although exercise therapy is considered an effective treatment in patients with knee osteoarthritis, the knowledge of which exercise modalities would be the most appropriate for this population is lacking. LLLT has been used as resource to increase the effects of physical therapy. However, the specific dose and treatment frequency need to be better defined. The findings from this randomised controlled trial will provide evidence of the efficacy or otherwise, of LLLT and stretching exercises in the management of knee OA symptoms. TRIAL REGISTRATION: NCT01738737 at ClinicalTrials.gov.
Methods: This study will involve 145 people aged 50-75 years with symptomatic-radiographic knee OA. It will consist of two types of treatments: Low-level laser therapy (LLLT) and stretching exercises. The patients will be randomly allocated to five groups LLLTACTIVE+Stretch, LLLTPLACEBO+Stretch, Stretch, LLLT and Control (n = 29 each). Treatment frequency will be three sessions/week for all active groups. LLLT will involve the use of a Gallium-Arsenide laser (904 nm, 40 milliwatts, 3 J/point, 27 J/knee) over 24 sessions for the monotherapy group and 9 sessions for the LLLT+Stretch groups. Stretching will consist of seven exercises completed over 24 sessions. The control group will receive a booklet. Participants will be treated for 2 months (Stretch, LLLT and Control groups) or 3 months (LLLT + Stretch groups). Participants and the outcome assessor will be blind to treatment allocation throughout the study. The primary outcome is pain measured by Visual Analogue Scale. Secondary outcomes include quality of life assessed by Western Ontario and McMaster Universities Arthritis Index, function by Lequesne Algofunctional Index, mobility by Timed Up and Go Test, KROM by goniometry of knee flexion and hamstring shortening by popliteal angle. The statistical method will follow the principles of per-protocol analysis.
Results: Although exercise therapy is considered an effective treatment in patients with knee osteoarthritis, the knowledge of which exercise modalities would be the most appropriate for this population is lacking. LLLT has been used as resource to increase the effects of physical therapy. However, the specific dose and treatment frequency need to be better defined. The findings from this randomised controlled trial will provide evidence of the efficacy or otherwise, of LLLT and stretching exercises in the management of knee OA symptoms.
Conclusions: NCT01738737 at ClinicalTrials.gov.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26369333
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26359814
o the Editor: Papulopustular rosacea (PPR) is traditionally treated with systemic and topical antibiotics or retinoids. Owing to flare-ups after discontinuation of therapy combined with frequent side effects, such as gastrointestinal discomfort, photosensitivity, and teratogenicity, alternative treatments need to be developed.1 No ideal laser treatment for PPR currently exists, and studies on the rejuvenation mode of long-pulsed 1064-nm neodymium:yttrium-aluminum-garnet laser (LPND) for PPR are lacking. This prospective case series evaluated the efficacy of the rejuvenation mode of LPND treatment for PPR. This study was approved by the ethics committee of the Catholic Medical Center Office of Human Research Protection Program (SC13RESE0196).
Thirty Korean patients with PPR were recruited in the Dermatology Department of Yeouido St Mary's Hospital from 2010 to 2013. Exclusion criteria were as follows: any previous treatment with laser or light-based devices; topical treatments with corticosteroids, metronidazole, or calcineurin inhibitors; and systemic treatments with antibiotics or retinoids during the prior 3 months. The patients were divided into 2 groups: 22 patients with mild- to moderate-grade PPR, according to Investigator Global Assessment, treated with laser only (group A); and 8 patients with severe-grade PPR treated with laser and doxycycline 100 mg twice daily (group B). All 30 patients underwent 3 treatment sessions, each with a 4-week interval. Patients used a topical anesthetic cream applied 30 minutes before laser treatment. Patients received full-face LPND (GentleMax; Candela, Wayland, MA) treatments at 40 to 50 J/cm2, with a pulse duration of 50 milliseconds, and a 10-mm spot size with a dynamic cooling device (Cryogen; Candela). Throughout this study, patients were instructed to use a moisturizer and a broad-spectrum sunscreen with an SPF of 30 or higher, and to avoid known triggering factors for rosacea.
Treatment efficacy was assessed using the 4-point severity grading system for rosacea at each visit and 4 weeks after the last treatment through blinded photographic evaluation by 3 dermatologists.2 Patients also evaluated their own rosacea symptoms at each visit (Table I).
| Characteristic | Group A (laser alone, n = 22) | Group B (laser + doxycycline, n = 8) |
|---|---|---|
| Age, y, mean (range) | 42.77 (23-62) | 43.13 (38-51) |
| Gender, n (%) | ||
| Female | 17 (77.3) | 7 (87.5) |
| Male | 5 (22.7) | 1 (12.5) |
| Fitzpatrick skin type, n (%) | ||
| IV | 2 (9) | 0 |
| V | 22 (91) | 8 (100) |
| Aggravation factor, n (%) | ||
| Heat | 18 (81.8) | 8 (100) |
| Emotional change | 13 (59.1) | 8 (100) |
| Exercise or bathing | 12 (54.5) | 8 (100) |
| Alcohol | 9 (40.9) | 6 (27.3) |
| Others | 20 (66.6) | 9 (30) |
| Investigator Global Assessment of baseline, n (%) | 0 | |
| Mild | 4 (13.3) | 0 |
| Moderate | 18 (60) | 0 |
Results and patient data are listed in Table I and Fig 1. Use of the rejuvenation mode of LPND significantly improved all outcome measures, including decreased papule/pustule activity and improved nontransient erythema score compared with baseline (Fig 2). In addition to these end points, LPND also had beneficial effects on clearance of symptoms such as transient erythema, pruritus, burning, and dryness. Excellent to good overall improvement was seen in 77.3% (17 of 22) of patients in group A and 87.5% (7 of 8) of patients in group B. In recent years, LPND has been widely used for photorejuvenation inducing destruction of telangiectases and reduction of wrinkles by dermal collagen remodeling.3, 4 Furthermore, through follicular ablation and selective photothermolysis, LPND has been reported to be effective for inflammatory lesions.5 We postulate multiple mechanisms of action of the rejuvenation mode of LPND to improve PPR. All treatments were well tolerated. No patients experienced purpura, hyperpigmentation, hypopigmentation, edema, or scarring. The adverse effects were minimal, and included temporary erythema and immediate mild pain, not interfering with the daily activities of the patients.
Fig 1
The clinical assessments by investigators (A) and patients (B) scored on the National Rosacea Society Expert Committee 4-point rosacea severity grading system (0, absent; 1, mild; 2, moderate; 3, severe) before treatment and at 4 weeks after 3 treatment session with long-pulsed neodymium:yttrium-aluminum-garnet laser.
Limitations of the study were the small number of subjects, absence of a control group, and the short follow-up period.
In conclusion, this study showed that mild to severe PPR responded favorably to the rejuvenation mode of LPND treatment. Thus, we suggest that this therapy could be a potentially effective monotherapy for patients with mild to moderate PPR, or as a combination therapy for severe PPR.
Abstract: PMID: 26183984 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26183984
Background: Low-level laser therapy (LLLT) continues to receive much attention in many clinical fields. Also, LLLT has been used to enhance the proliferation of various cell lines, including stem cells. This study investigated the effect of LLLT on human adipose-derived stem cells (ADSCs) through in vitro and in vivo studies.
Abstract: Abstract BACKGROUND: Low-level laser therapy (LLLT) continues to receive much attention in many clinical fields. Also, LLLT has been used to enhance the proliferation of various cell lines, including stem cells. This study investigated the effect of LLLT on human adipose-derived stem cells (ADSCs) through in vitro and in vivo studies. METHODS: Low-level laser irradiation of cultured ADSCs was performed using a 830 nm Ga-Al-As (gallium-aluminum-arsenide) laser. Then, proliferation of ADSCs was quantified by a cell counting kit-8. In the in vivo study, irradiated ADSCs or non-irradiated ADSCs were transplanted, and then, low-level laser irradiation of each rat was performed as per the protocol. Cell viability was quantified by immunofluorescent staining using the human mitochondria antibody. RESULTS: In the in vitro study, the laser-irradiated groups showed an increase in absorbance compared to the control group. Also, in the in vivo study, there was a significant increase in the number of human ADSCs in the laser-irradiated groups compared to the control group (p < 0.001). CONCLUSIONS: Our study showed that LLLT could enhance the proliferation and viability of ADSCs. The ADSCs enhanced by LLLT could be applied in various clinical fields. With the use of LLLT, the proliferation and viability of various cells can be enhanced, besides ADSCs. NO LEVEL ASSIGNED: This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors http://www.springer.com/00266 .
Methods: Low-level laser irradiation of cultured ADSCs was performed using a 830 nm Ga-Al-As (gallium-aluminum-arsenide) laser. Then, proliferation of ADSCs was quantified by a cell counting kit-8. In the in vivo study, irradiated ADSCs or non-irradiated ADSCs were transplanted, and then, low-level laser irradiation of each rat was performed as per the protocol. Cell viability was quantified by immunofluorescent staining using the human mitochondria antibody.
Results: In the in vitro study, the laser-irradiated groups showed an increase in absorbance compared to the control group. Also, in the in vivo study, there was a significant increase in the number of human ADSCs in the laser-irradiated groups compared to the control group (p < 0.001).
Conclusions: Our study showed that LLLT could enhance the proliferation and viability of ADSCs. The ADSCs enhanced by LLLT could be applied in various clinical fields. With the use of LLLT, the proliferation and viability of various cells can be enhanced, besides ADSCs.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/26183254
Abstract: PMID: 25958474 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Following the split-mouth design, self-tapping implants n = 44) were inserted in the posterior maxilla of 12 patients. One jaw side randomly received LLLT (test group), while the other side was placebo (control group). For LLLT, a 637 nm gallium-aluminum-arsenide (GaAlAs) laser (Medicolaser 637, Technoline, Belgrade, Serbia) with an output power of 40 mW and continuous wave was used. Low-level laser treatment was performed immediately after the surgery and then repeated every day in the following 7 days. The total irradiation dose per treatment was 6.26 J/cm2 per implant. The study outcomes were: implant stability, alkaline-phosphatase (ALP) activity and early implant success rate. The follow-up took 6 weeks.
Results: Irradiated implants achieved a higher stability compared with controls during the entire follow-up and the difference reached significance in the 5th postoperative week (paired t-test, p = 0.030). The difference in ALP activity between the groups was insignificant in any observation point (paired t-test, p > 0.05). The early implant success rate was 100%, regardless of LLLT usage.
Conclusions: LLLT applied daily during the first postoperative week expressed no significant influence on the osseointegration of self-tapping implants placed into low density bone of the posterior maxilla. Placement of self-tapping macro-designed implants into low density bone could be a predictable therapeutic procedure with a high early success rate regardless of LLLT usage.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25958474
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25844681
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25806375
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25773417
Abstract: PMID: 25738176 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Literature search was performed on PubMed, Scopus, Web of Science and Cochrane databases up to July 2014. Inclusion criteria were: (1) RCTs; (2) orthodontic therapy on permanent dentition; (3) application of adjunctive surgical or non-surgical procedures for accelerating OTM; (4) measurement of tooth movement. The primary outcome measure was tooth movement expressed as cumulative tooth movement (CTM), rate of tooth movement (RTM) or time of tooth movement (TTM). Pain and discomfort, periodontal health, anchorage loss, bone and root changes, and undesired tooth movement were evaluated as secondary outcomes.
Results: Literature research identified 184 studies. After screening of titles, abstracts and full-text studies, fifteen fulfilled the inclusion criteria and were included in this review. Six of the included studies investigated the effect of corticotomies, one of interseptal bone reduction, four of lowlevel laser therapy (LLLT), three of intraoral/extraoral devices releasing extracorporeal shock waves (ESWT), pulsed electromagnetic field (PEMF) and electrical current, respectively, and one of injected substances (relaxin) as an adjunct to OT. Three studies resulted of high methodological quality, six of medium, and six of low quality. Interseptal bone reduction was reported to increase RTM during the first 2 months (P = 0.002) and CTM at 3 months (P = 0.003). Studies investigating corticotomy reported significantly increased RTM (up to 2.3 times) during the first months after intervention, whereas results on TTM and CTM were quite controversial ranging from non-significant to highly significant (up to three times of TTM increase). The heterogeneity between studies investigating corticotomy could not allow for quantitative synthesis of the findings. Out of four studies investigating LLLT three reported positive effect on OT. Due to inadequate statistical analysis of data from original articles, results could not be summarised in meta-analyses. Effects of both electrical current devices and PEMF devices on CTM were reported to be larger on the experimental sides than on the control sides (P < 0.001). The other interventions were reported to be of no statistical or clinical relevance.
Conclusions: In the short term, corticotomy can accelerate OTM whereas long-term effects are questionable, thus no firm conclusions can be made on its efficacy and benefit of clinical use. There is some evidence that LLLT can slightly accelerate OTM but this result is not significant and the effect estimated is not clinically relevant. The very limited research-based evidence suggesting beneficial effects of interseptal bone reduction, electrical current and PEMF on OTM does not allow for solid conclusions. More high quality clinical research is required in order to estimate the efficacy of adjunctive interventions on accelerating OTM and their potential clinical use.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25738176
Abstract: PMID: 25611979 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25611979
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25499403
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25402725
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25368481
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25219714
Abstract: PMID: 25198641 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25198641
Abstract: PMID: 25134310 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25134310
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25084500
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25014468
Abstract: PMID: 25003752 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/25003752
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24992270
Abstract: PMID: 24964567 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: The articles published until May 2013 were obtained from the Medline/PubMed online database, using following search terms and key words: "laser therapy" and "pemphigus vulgaris", "low-level laser irradiation" and "pemphigus vulgaris", "lasers" and "pemphigus vulgaris" and "pemphigus vulgaris".
Results: Low-level laser therapy could result in immediate and significant analgesia and improved wound healing within the observation period and follow-up. Furthermore, a decrease in patients' discomfort as well as the absence of recurrence of the pemphigus vulgaris lesions has been claimed.
Conclusions: Even though available literature suggests that low-level laser therapy can be efficiently used in treatment of oral pemphigus vulgaris, either independently or as a part of combined therapy approach, these results should be interpreted with caution since there are no solid evidence-based proofs to provide the guidelines for the treatment of pemphigus vulgaris with low-level laser therapy. Therefore, further long-term randomized controlled clinical studies are necessary in order to give any solid recommendations on the use of low-level laser therapy in the treatment of pemphigus vulgaris.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24964567
Abstract: PMID: 24945286 [PubMed - indexed for MEDLINE] PMCID: PMC4273713 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Methods: To assess the efficacy of low-level laser therapy added to IVRA for improving pain related to surgical fixation of distal radius fractures.
Results: The present double-blinded, placebo-controlled, randomized clinical trial involved 48 patients who were undergoing surgical fixation of distal radius fractures. Participants were randomly assigned to either an intervention group (n=24), who received 808 nm laser irradiation as 4 J�point for 20 s over ipsilateral three nerve roots in the cervical region corresponding to C5-C8 vertebrae, and 808 nm laser irradiation as 0.1 J�cm2 for 5 min in a tangential scanning mode over the affected extremity; or a control group (n=24), who underwent the same protocol and timing of laser probe application with the laser switched off. Both groups received the same IVRA protocol using 2% lidocaine.
Conclusions: The mean visual analogue scale scores were significantly lower in the laser-assisted group than in the lidocaine-only group on all measurements during and after operation (P<0.05). The mean time to the first need for fentanyl administration during the operation was longer in the laser group (P=0.04). The total amount of fentanyl administered to patients was significantly lower in the laser-assisted group (P=0.003). The laser group needed significantly less pethidine for pain relief (P=0.001) and at a later time (P=0.002) compared with the lidocaine-only group. There was no difference between the groups in terms of mean arterial pressure and heart rate.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24945286
Abstract: PMID: 24812743 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24812743
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24703139
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24685879
Abstract: PMID: 24660647 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Each hemiface of 19 subjects was randomized to receive intervention, in a total of 116 sensitive points. Pain was measured at baseline and time intervals of 24 hours, 30 days, 90 days, and 180 days after treatment. Irradiation of 4 J/cm2 in the temporomandibular joints and 8 J/cm(2) in the muscles was used in three sessions.
Results: Both treatments had statistically significant results (P<0.001); there was statistical difference between them at 180 days in favor of the infrared laser (P=0.039). There was improvement in 24 hours, which extended up to 180 days in both groups.
Conclusions: Both lasers are effective in the treatment and remission of TMD symptoms.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24660647
Abstract: PMID: 24660645 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: In a double-blind clinical trial, 20 patients with TMJ osteoarthritis were randomly divided into laser and placebo groups. The patients in the laser group received irradiation from an 810 nm low-level laser (Peak power 80 W, average power 50 mW, 1500 Hz, 1 micro s pulse width, 120 seconds, 6 J, 3.4 J/cm(2) per point), which was applied on four points around the TMJs and on painful muscles three times a week for 4 weeks. In the placebo group, the treatment was the same as that in the laser group, but with laser simulation. The patients were evaluated before laser therapy (T1), after 6 (T2) and 12 (T3) laser applications and 1 month after the last application (T4), and the amount of mouth opening and the pain intensity were recorded.
Results: No significant differences were found in mouth opening either between the study groups or between the different evaluation times in each group (P>0.05). There was no significant difference in pain symptoms of the masticatory muscles and TMJ between the laser and the placebo groups (P>0.05), but some significant within-group improvements were present for Visual Analogue Scale (VAS) scores of the body of the masseter and TMJ in both groups.
Conclusions: LLLT using the present laser parameters was no more effective than the placebo treatment for reducing pain and improving mouth opening in patients with TMJ osteoarthritis.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24660645
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24661051
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24625134
Abstract: PMID: 24595582 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24595582
Abstract: PMID: 24384987 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Case report.
Results: Class 4 therapeutic laser treatment was applied with a dual wavelength GaAlAs (810 nm), GaAl (980 nm) laser, 2 to 4 W, 50% duty cycle, 10 Hz pulse active phase, 2.5 cm diameter aperture, scanning technique with skin contact, 10-minute treatment, 600 to 1200 J total, energy density of 3.5 to 7.1 J/cm average per session, and power density from 0.41 to 0.82 W/cm for 8 treatments. Outcome measures included the Neuropathy Pain Scale Questionnaire as the primary outcome measure, with the Numeric Pain Scale and total area of allodynia touch sensitivity as secondary outcome measurements.
Conclusions: The author reports a case of PHN of 15-year duration resistant to prior interventions. Weekly laser therapy treatment over 8 weeks resulted in reduced 0 to 10 Numeric Pain Scale score from 8 to 0, Neuropathy Pain Scale Questionnaire total score from 39 to 4, and allodynia over a 60 cm surface area of the upper trunk and posterior arm totally resolved, with resolution continued at 14-month follow-up.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24384987
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24372626
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24359266
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24353781
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24353764
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24347912
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24261728
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24237486
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24237619
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24232862
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24219300
Abstract: PMID: 24173246 [PubMed - indexed for MEDLINE] Free full text Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24173246
Abstract: PMID: 24153107 [PubMed - indexed for MEDLINE] PMCID: PMC3854767 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24153107
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24131068
Abstract: PMID: 24110550 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24110550
Abstract: PMID: 24085055 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Two patients underwent surgery to remove facial skin cancer tumors. The resulting scars after reconstruction of these skin cancer defects on the left cheek (Case 1) and right cheek (Case 2) each received 3 treatments with a fractional ablative laser device (ProFractional-XC, Sciton, Inc., Palo Alto, CA). Treatments were spaced about 1 month apart. Topical anesthetic cream applied 1 hour before treatment minimized patient discomfort during the procedure. Treatment depths ranged from 150 to 200 microns, 2 passes were performed, and coverage per pass was typically 22% and then 11% in the coagulation mode. Results were evaluated by digital photography before the initial treatment, approximately 4-5 weeks after each of the 3 treatments, and at approximately 7 months after the surgical procedures.
Results: The fractional Er:YAG laser device significantly improved postsurgical scar lines in each patient without significant adverse effects. Prior to the laser sessions, these scars demonstrated hypopigmentation, hyperpigmentation, neovascularization, or diminished pore structures compared to the surrounding skin. These pigmentary, vascular or textural issues were all significantly improved by the fractional ablative Er:YAG laser.
Conclusions: The ablative fractional laser device of the present report safely minimizes and improves facial scars demonstrating not only textural alterations but also some pigmentary and vascular changes after reconstruction of skin cancer defects.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24085055
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24049935
Abstract: PMID: 24000332 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Fifteen bovine incisors were selected and the roots removed. Crowns were sectioned into four pieces, resulting in 60 samples that were individually embedded in polyester resin (n = 15) and ground to plane the enamel and expose the dentin. The bonding site was delimited and samples were randomly assigned according to the method of cavity preparation: Er:YAG laser (250 mJ/4 Hz) or high-speed handpiece (diamond bur #2096). Samples were fixed to a metallic device, where glass-ionomer cement (GIC) cylinders were prepared. Subsequently, they were subdivided according to the duration of water storage (WS) and number of thermocycles (TCs) - 24 h WS/no TCs and 6 months WS/12,000 TCs - and subjected to a shear bond strength test (500 N at 0.5 mm/min).
Results: The duration of water storage and number of thermocycles tested had no statistically significant effect on the shear bond strength to laser-irradiated dentin (p > 0.05). For bur-prepared substrate, the long-term degradation process promoted a decrease in shear bond strength values (p < 0.05).
Conclusions: Long-term water storage and thermocycling did not affect shear bond strength of glass-ionomer cement bonded to Er:YAG laser-prepared dentin.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24000332
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23883266
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23700692
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23619448
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23573711
Abstract: PMID: 23464882 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23464882
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23452447
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23351678
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23312184
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23312183
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23304989
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23176844
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23132724
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23094278
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23049388
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23019847
Background: This review aimed to identify the efficacy of low-level laser therapy (LLLT) in the management of orthodontic pain. This systematic review and meta-analysis was carried out in accordance with Cochrane Handbook and the PRISMA statement. An extensive literature search for RCTs, quasi-RCTs, and CCTs was performed through CENTRAL, PubMed, Embase, Medline, CNKI, and CBM up to October 2011. Risk of bias assessment was performed via referring to the Cochrane tool for risk of bias assessment. Meta-analysis was implemented using Review Manager 5.1. As a result, four RCTs, two quasi-RCTs, and two CCTs were selected from 152 relevant studies, including 641 patients from six countries. The meta-analysis demonstrated that 24% risk of incidence of pain was reduced by LLLT (RR = 0.76, 95% CI range 0.63-0.92, P = 0.006). In addition, compared to the control group, LLLT brought forward "the most painful day" (MD = -0.42, 95% CI range -0.74- -0.10, P = 0.009). Furthermore, the LLLT group also implied a trend of earlier end of pain compared with the control group (MD = -1.37, 95% CI range -3.37-0.64, P = 0.18) and the pseudo-laser group (MD = -1.04, 95% CI range -4.22-2.15, P = 0.52). However, because of the methodological shortcomings and risk of bias of included trials, LLLT was proved with limited evidence in delaying pain onset and reducing pain intensity. In the future, larger and better-designed RCTs will be required to provide clearer recommendations.
Abstract: Abstract This review aimed to identify the efficacy of low-level laser therapy (LLLT) in the management of orthodontic pain. This systematic review and meta-analysis was carried out in accordance with Cochrane Handbook and the PRISMA statement. An extensive literature search for RCTs, quasi-RCTs, and CCTs was performed through CENTRAL, PubMed, Embase, Medline, CNKI, and CBM up to October 2011. Risk of bias assessment was performed via referring to the Cochrane tool for risk of bias assessment. Meta-analysis was implemented using Review Manager 5.1. As a result, four RCTs, two quasi-RCTs, and two CCTs were selected from 152 relevant studies, including 641 patients from six countries. The meta-analysis demonstrated that 24% risk of incidence of pain was reduced by LLLT (RR = 0.76, 95% CI range 0.63-0.92, P = 0.006). In addition, compared to the control group, LLLT brought forward "the most painful day" (MD = -0.42, 95% CI range -0.74- -0.10, P = 0.009). Furthermore, the LLLT group also implied a trend of earlier end of pain compared with the control group (MD = -1.37, 95% CI range -3.37-0.64, P = 0.18) and the pseudo-laser group (MD = -1.04, 95% CI range -4.22-2.15, P = 0.52). However, because of the methodological shortcomings and risk of bias of included trials, LLLT was proved with limited evidence in delaying pain onset and reducing pain intensity. In the future, larger and better-designed RCTs will be required to provide clearer recommendations.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23001570
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22973818
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22861497
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22748981
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22706567
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22681785
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22577684
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22484553
Abstract: PMID: 22468172 [PubMed] PMCID: PMC3315881 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Methods: Randomized, double-blind study whereby healthy subjects (N=40) with a body mass index of 20 to 35kg/m(2) received three 20-minute low-level laser therapy (N=20) or sham treatments (N=20) each week for two weeks.
Results: Upper arm circumference was measured after three and six treatments and two weeks post-treatment. Primary success criterion was the proportion of subjects achieving a combined reduction in arm circumference of ≥1.25cm measured at three equally spaced points between the elbow and the shoulder. Secondary outcomes included total measurement change at each time point and subjective satisfaction ratings.
Conclusions: After six treatments, the low-level laser therapy group showed a combined reduction in arm circumference of 3.7cm versus 0.2cm in the sham treatment group (p<0.0001). Significantly more subjects in the low-level laser therapy group (N=12; 60%) achieved ≥1.5cm total decrease in upper arm circumference versus sham-treated subjects (N=0; 0%) (p<0.0005). Low-level laser therapy treatment resulted in a combined reduction in arm circumference of 2.2cm after three treatments and 3.7cm after six treatments (for each, p<0.0001) indicating a progressive and cumulative treatment effect. Body mass index remained unchanged for all subjects. A significantly greater number of subjects in the low-level laser therapy treatment group were satisfied with their results (p<0.05), believed their upper arm appearance improved (p<0.0005), and indicated the results exceeded expectations (p<0.05). The treatments were painless and no adverse events were reported.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22468172
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24610982
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22176508
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22145236
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22145235
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22134557
Background: An update of a Dutch physiotherapy practice guideline in Hip and Knee Osteoarthritis (HKOA) was made, based on current evidence and best practice.
Abstract: Abstract BACKGROUND: An update of a Dutch physiotherapy practice guideline in Hip and Knee Osteoarthritis (HKOA) was made, based on current evidence and best practice. METHODS: A guideline steering committee, comprising 10 expert physiotherapists, selected topics concerning the guideline chapters: initial assessment, treatment and evaluation. With respect to treatment a systematic literature search was performed using various databases, and the evidence was graded (1-4). For the initial assessment and evaluation mainly review papers and textbooks were used. Based on evidence and expert opinion, recommendations were formulated. A first draft of the guideline was reviewed by 17 experts from different professional backgrounds. A second draft was field-tested by 45 physiotherapists. RESULTS: In total 11 topics were selected. For the initial assessment, three recommendations were formulated, pertaining to history taking, red flags, and formulating treatment goals. Concerning treatment, 7 recommendations were formulated; (supervised) exercise therapy, education and self management interventions, a combination of exercise and manual therapy, postoperative exercise therapy and taping of the patella were recommended. Balneotherapy and hydrotherapy in HKOA, and thermotherapy, TENS, and Continuous Passive Motion in knee OA were neither recommended nor discouraged. Massage therapy, ultrasound, electrotherapy, electromagnetic field, Low Level Laser Therapy, preoperative physiotherapy and education could not be recommended. For the evaluation of treatment goals the following measurement instruments were recommended: Lequesne index, Western Ontario and McMaster Universities osteoarthritis index, Hip disability and Osteoarthritis Outcome Score and Knee injury and Osteoarthritis Outcome Score, 6-minute walktest, Timed Up and Go test, Patient Specific Complaint list, Visual Analoge Scale for pain, Intermittent and Constant OsteoArthritis Pain Questionnaire, goniometry, Medical Research Council for strength, handheld dynamometer. CONCLUSIONS: This update of a Dutch physiotherapy practice guideline on HKOA included 11 recommendations on the initial assessment, treatment and evaluation. The implementation of the guideline in clinical practice needs further evaluation.
Methods: A guideline steering committee, comprising 10 expert physiotherapists, selected topics concerning the guideline chapters: initial assessment, treatment and evaluation. With respect to treatment a systematic literature search was performed using various databases, and the evidence was graded (1-4). For the initial assessment and evaluation mainly review papers and textbooks were used. Based on evidence and expert opinion, recommendations were formulated. A first draft of the guideline was reviewed by 17 experts from different professional backgrounds. A second draft was field-tested by 45 physiotherapists.
Results: In total 11 topics were selected. For the initial assessment, three recommendations were formulated, pertaining to history taking, red flags, and formulating treatment goals. Concerning treatment, 7 recommendations were formulated; (supervised) exercise therapy, education and self management interventions, a combination of exercise and manual therapy, postoperative exercise therapy and taping of the patella were recommended. Balneotherapy and hydrotherapy in HKOA, and thermotherapy, TENS, and Continuous Passive Motion in knee OA were neither recommended nor discouraged. Massage therapy, ultrasound, electrotherapy, electromagnetic field, Low Level Laser Therapy, preoperative physiotherapy and education could not be recommended. For the evaluation of treatment goals the following measurement instruments were recommended: Lequesne index, Western Ontario and McMaster Universities osteoarthritis index, Hip disability and Osteoarthritis Outcome Score and Knee injury and Osteoarthritis Outcome Score, 6-minute walktest, Timed Up and Go test, Patient Specific Complaint list, Visual Analoge Scale for pain, Intermittent and Constant OsteoArthritis Pain Questionnaire, goniometry, Medical Research Council for strength, handheld dynamometer.
Conclusions: This update of a Dutch physiotherapy practice guideline on HKOA included 11 recommendations on the initial assessment, treatment and evaluation. The implementation of the guideline in clinical practice needs further evaluation.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22113602
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22107485
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22092942
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22086666
Abstract: PMID: 22027112 [PubMed] PMCID: PMC3206566 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Methods: A 62-year-old male truck driver (of 35 years) presented to a chiropractic clinic with pain and stiffness along the third metacarpal and MCP joint of the left hand. Examination revealed severe pain and limited flexion at the third MCP joint. Bilateral radiographs demonstrated severe osteoarthritis (OA) of this joint in the left (nondominant) hand and mild-to-moderate (asymptomatic) OA in the same joint on the right. Results of laboratory blood tests were unremarkable for metabolic, inflammatory, or infectious joint disease.
Results: The patient was diagnosed with bilateral, third MCP joint OA associated with manual labor. He was treated unsuccessfully with a short course of low-level laser therapy, MCP joint mobilization, and hand-stretching exercises. After 3½ years, the patient continues to work despite ongoing and worsening symptoms. Three serial left hand radiographs are presented, highlighting the progressive nature of this arthropathy.
Conclusions: The differential diagnosis in patients presenting with manual labor MCP joint OA should include hemochromatosis and calcium pyrophosphate dihydrate crystal deposition disease. Because of the increased risk of serious systemic disease, it is imperative that these latter 2 disorders are ruled out before the former is diagnosed.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22027112
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/22006130
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21997801
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21895530
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21801008
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21790846
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21786023
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21768476
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21721284
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21711410
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21707830
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21707831
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21631379
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21615600
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21597949
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21574478
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21497261
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21492302
Abstract: PMID: 21491809 [PubMed - indexed for MEDLINE] Free full text Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21491809
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21492001
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21457396
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21410824
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21375456
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21324032
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21272121
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/24155532
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21142728
Abstract: PMID: 21122053 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: To report a clinical case of surgical lip Fordyce granule excision in a 19-year-old male.
Results: Fordyce granules were excised using a high-power diode laser (gallium arsenide [GaAs], Diode Vision®, MDL, 10 Dental Laser Unit, GmbH, Lower Saxony, Germany) with wavelength emission at 980 ± 10 nm, in a continuous wave mode, pulse width of 0.5 μs, fiber optic delivery system of 400 μm in diameter, at 2.5 W. Subsequently, low-intensity laser therapy was applied (gallium-aluminum-arsenide [GaAlAs], at 670 nm, 50 mW, at 4 J/cm(2); Dentoflex®, São Paulo, Brazil] in order to stimulate a faster wound tissue-healing process and less postoperative pain and inflammation.
Conclusions: The excellent esthetic result demonstrated the effectiveness of both high- and low-intensity laser therapy on the excision of Fordyce granules.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21122053
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21124109
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21091418
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/21070473
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20972593
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20965023
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20868377
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20714843
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20704500
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20614767
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20603704
Abstract: PMID: 20509817 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20509817
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20466181
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20384735
Abstract: PMID: 20359282 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: Different laser systems (diode laser at 810 nm; Er,Cr:YSGG laser at 2780 nm; Erbium:YAG laser at 2940 nm) were used, both for soft tissue surgery and enamel etching, and for biostimulating effect. These wavelengths were used with different parameters for each case, according to international current studies in view of minimally invasive therapy.
Results: The cases reported showed very quick and good healing of the laser treated tissues. These treatments, necessary for the orthodontic therapy or for its completion, become extremely simple, safe and rapid and the orthodontic specialist can perform them himself.
Conclusions: The laser technique is very effective in many operative and surgical procedures during orthodontic therapy. Further studies are however necessary to set the treatment protocols in orthodontic biostimulation.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20359282
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20331349
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20308196
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20193864
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20193863
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20191447
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20189016
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20189015
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20187893
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20155297
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20155297
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20083687
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20041520
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20035607
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/20035605
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19938271
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19912730
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19878032
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19841394
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19835348
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19835348
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19796255
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19777973
Abstract: PMID: 19717066 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19717066
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19694512
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19682001
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19682000
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19681988
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19630279
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19566054
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19549177
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19546509
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19438681
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19382843
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19351022
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19323339
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19288600
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19288599
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19205062
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19177664
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19180899
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19175057
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19175055
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19150293
Abstract: PMID: 23074533 [PubMed] PMCID: PMC3377577 Free PMC Article Share on Facebook Share on Twitter Share on Google+
Methods: The Medical Advisory Secretariat (MAS) conducted a systematic review on interventions used to treat pressure ulcers in order to answer the following questions: Do currently available interventions for the treatment of pressure ulcers increase the healing rate of pressure ulcers compared with standard care, a placebo, or other similar interventions?Within each category of intervention, which one is most effective in promoting the healing of existing pressure ulcers?
Results: A pressure ulcer is a localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in conjunction with shear and/or friction. Many areas of the body, especially the sacrum and the heel, are prone to the development of pressure ulcers. People with impaired mobility (e.g., stroke or spinal cord injury patients) are most vulnerable to pressure ulcers. Other factors that predispose people to pressure ulcer formation are poor nutrition, poor sensation, urinary and fecal incontinence, and poor overall physical and mental health. The prevalence of pressure ulcers in Ontario has been estimated to range from a median of 22.1% in community settings to a median of 29.9% in nonacute care facilities. Pressure ulcers have been shown to increase the risk of mortality among geriatric patients by as much as 400%, to increase the frequency and duration of hospitalization, and to decrease the quality of life of affected patients. The cost of treating pressure ulcers has been estimated at approximately $9,000 (Cdn) per patient per month in the community setting. Considering the high prevalence of pressure ulcers in the Ontario health care system, the total cost of treating pressure ulcers is substantial.
Conclusions: Wounds normally heal in 3 phases (inflammatory phase, a proliferative phase of new tissue and matrix formation, and a remodelling phase). However, pressure ulcers often fail to progress past the inflammatory stage. Current practice for treating pressure ulcers includes treating the underlying causes, debridement to remove necrotic tissues and contaminated tissues, dressings to provide a moist wound environment and to manage exudates, devices and frequent turning of patients to provide pressure relief, topical applications of biologic agents, and nutritional support to correct nutritional deficiencies. A variety of adjunctive physical therapies are also in use.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/23074533
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19086104
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19076183
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19069805
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/19069795
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18936391
Abstract: PMID: 18789060 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18789060
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18754723
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18687055
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18618360
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18490694
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18459516
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18384612
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18335647
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18312432
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18277406
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18248166
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18190544
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18177408
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18158757
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18088730
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18076622
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/18053032
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17975963
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17970140
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17647310
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17632868
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17619433
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17619431
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17603866
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17598853
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17596612
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17576934
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17563978
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17515508
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17515508
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17508850
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17508608
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17419509
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17402628
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17296350
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17272683
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17278698
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17236670
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17214689
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17214688
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17201227
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17163958
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17144562
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17124850
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17087041
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17083598
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/17055394
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16995282
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16942458
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16942437
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16875483
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16858330
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16752818
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16710285
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16697111
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16640698
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16635693
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16568446
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16503792
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16485827
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16442058
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16422262
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16404921
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16388763
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16356141
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16350436
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16328096
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16304923
Abstract: PMID: 16281465 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: CAPT is conducted in 23 clinical centers and in three central resource centers. The primary outcome measure is change in visual acuity; secondary outcomes include the incidence of complications of AMD, changes in other measures of visual functioning and vision-related quality of life. In total, 1052 patients with two high-risk eyes were enrolled. One eye was randomized to receive laser treatment and the other eye to observation. All patients were treated immediately after randomization and again at 12 months, dependent on clinical status. All patients are followed via study visits and telephone calls for a minimum of five years. Study visit procedures include established tests of visual function conducted by examiners masked to the treatment assignment of each eye, a biomicroscopic examination by CAPTophthalmologists, and photographs of each eye taken according to protocol and assessed by masked graders in a centralized Photograph Reading Center.
Results: This paper describes the CAPT study, including study rationale, operational structure, and measures implemented to ensure standardization of assessments, adherence to protocol, quality assurance, and maintaining follow-up. Several features related to study design and procedures that are specific to CAPT are highlighted, including clinic selection and judgements regarding patient eligibility.
Conclusions: An intervention that can reduce the risk of advanced AMD by 30% in the eyes of people with two high-risk eyes may halve the rate of bilateral blindness from AMD. It would also yield substantial savings in expenditures devoted to treating advanced AMD and the disability it causes, and enhance the quality of life for people at risk.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16281465
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16262572
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16251087
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16234556
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16206961
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16144489
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16108041
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/16060283
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15967912
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15962599
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15957498
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15916378
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15864963
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15822776
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15801642
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15773387
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15759854
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15750548
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15727104
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15724009
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15719728
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15714675
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15675751
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15654166
Abstract: PMID: 15545099 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Methods: To describe the use of a 585-nm PDL for the treatment of a retracted and atrophic facial scar.
Results: We report the case of a 26-year-old patient who presented with a retracted facial scar following surgical excision of an aggressive benign tumor. Treatment was carried out using the 585-nm PDL.
Conclusions: Treatment of the scar using two low-level PDL therapies significantly altered the appearance of the scar and augmentation of the retracted defect was avoided.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15545099
Abstract: PMID: 15509188 [PubMed - indexed for MEDLINE] Free full text Share on Facebook Share on Twitter Share on Google+
Methods: Using Cochrane Collaboration methods, the Ottawa Methods Group identified and synthesized evidence from comparative controlled trials. The group then formed an expert panel, which developed a set of criteria for grading the strength of the evidence and the recommendation. Patient-important outcomes were determined through consensus, provided that these outcomes were assessed with a validated and reliable scale.
Results: The Ottawa Panel developed 8 positive recommendations of clinical benefit. Lack of evidence meant that the panel could not gauge the efficacy of electrical stimulation.
Conclusions: The Ottawa Panel recommends the use of low-level laser therapy, therapeutic ultrasound, thermotherapy, electrical stimulation, and transcutaneous electrical nerve stimulation for the management of rheumatoid arthritis.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15509188
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15471404
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15449674
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15383510
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15216785
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15148093
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15052842
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14997890
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14966584
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14974136
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14753013
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14724973
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14677336
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14660275
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14650142
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/14584438
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12945136
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12928824
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12852022
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12852009
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12782829
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12722360
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12698702
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12592899
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12564236
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12559891
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12559890
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12549280
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12533109
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12533169
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12532595
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12455132
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12400143
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12397943
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12227049
Abstract: PMID: 12149787 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12149787
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12046535
Abstract: PMID: 12056574 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/12056574
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11998760
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11976982
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11913929
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11726330
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11710855
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11696955
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11561309
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/11550382
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/10623342
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/9414066
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/9470287
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/2103917
Abstract: PMID: 3948803 [PubMed - indexed for MEDLINE] Share on Facebook Share on Twitter Share on Google+
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/3948803
At the Lyme Laser center of New England, we have successfully cared for hundreds of Acute and Chronic Lyme patients. Our research has proven that all Lyme patients suffer from undiagnosed environmental toxicity that severely suppresses their immune function. Because of their compromised immune function, Lyme patients always have underlying causes that go undiagnosed and overlooked. It is just as important to be able to find out what these issues are and address them as well as the Lyme itself. This, along with our Lyme Specific Lasers and proprietary Supplements, is one of the reasons why our success rate is better than 90 percent.
OUR TREATMENT PROTOCOLS FOR LYME DISEASE
The protocol that we have developed has been used for years on hundreds of Lyme patients with great success. Every protocol is specific to each Lyme patient. In fact, our success rate is better than 90 percent.
The #1 reason for our success is the use of cold laser therapy also known as low level lasers. Reason #2 is that our in-depth questionnaire finds the secondary and tertiary causes which allows us to address them as well as the Lyme.
“This condition is better termed Lyme MSIDS, short for Multiple Systemic Infectious Disease Syndrome. MSIDS is like Pandora’s Box because it includes many infections, co-infections and secondary infections. Treatment should be tailored to each patient individually.” Richard Horowitz, MD and author of the book, Why Can’t I Get Better? Solving the Mystery of Lyme and Chronic Disease.
We only use safe, natural, non-invasive modalities, techniques and supplements including, but not limited to:
Whole-body Laser
Specific Lyme frequency lasers
Ionic Detox
Oscillation
Anatomotor
Specific nutrition and proprietary supplements
Evaluation of all external factors including chemicals and ionizing radiation
Addressing stress issues
Addressing parasitic, bacterial, viral infections and fungus
Addressing other lifestyle factors including EMF, microwaves, wireless technologies, etc.
SPECIFIC LOW LEVEL/COLD LASERS
The wave of the future is these new lasers. Dr. Wine has developed protocols and different types of these specific lasers since 1995. These non-invasive lasers have the ability to significantly accelerate and enhance the body’s natural defense and repair components to increase your health potential. Cold lasers deliver the required energy directly to the cells which enhance their ability to produce ATP (adenosine-tri-phosphate) – which is necessary for optimal function, cell repair and regeneration, healing, weight loss and endorphin production, which are the body’s natural pain killers.
Laser therapy has a direct effect on immunity status by stimulation of immunoglobins and lymphocytes. Laser light is absorbed by chromophones (molecule enzymes) that react to laser light. The enzyme flavomono-nucleotide is activated and starts the production of ATP, which is the major carrier of cell energy and the energy source for all chemical reactions in the cells.
Original Source: http://www.lymelasercentersofnewengland.com/
ColdLasers recently sold a system to Dr. Wie Chen. He has numerous publications, including several with Dr Hamblin and others and is a leading researcher in the use of higher power lasers for cancer treatment. During the interview, he reviewed how he uses high powered laser to thermally destroy cancer followed up by a special program to promote normal healing. This is done by increasing the tissue temperature to around 60 degree C (140 degree F). This is not using the laser to cut out the cancer but to thermally destroy the damaged cells.
During the interview, I ask if non-thermal dosages of LLLT can help cancer grow. He said that they do see growth in the cancer in tissue sample with non-thermal (low intensity) LLLT.
We are adding this “non-published” information to the library as a contrary position to the studies that show there is no interaction with cancer and LLLT. We have heard from some manufacturer, that LLLT only grows healthy cells but Dr Chen’s research conflicts with this information.
Original Source: http://www.ncbi.nlm.nih.gov/pubmed/15154357
This tool is a searchable collection of technical publications, books, videos and other resources about the use of lasers and light for PhotoBioModulation (PBM). Enter a keyword above or see some of our favorite queries below.
Here are some of our favorite queries:
- Information for Beginners
- Best wavelength for PBM (980nm vs. 810nm vs 660nm...)
- Compare Lasers and LEDs
- Pulsing versus Continuous Wave (CW)
- Dosage: The best practices for dosage
- How NOT to promote laser therapy (by Turner and Hode)
- Great summary of positive double blind studies
- Whole Body Systems
- See all videos
- See all books about laser therapy and cold lasers
- Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing Athletic Performance
- Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Intro to laser acupuncture
- Traumatic brain injury research
- Soft Tissue
- Autoimmune
- Lyme's Disease
- Parkinson's research
- Alzheimer's research
- Hashimoto's research
- Covid
- Contraindications
- LLLT and Cancer
All the resources include links to the original source so we are not making any statement about the use of lasers for treating non-FDA cleared application, we are simple summarizing what others have said.
Where every possible, we have included a link to the orginal publication.
This tool uses a broad match query so:
- It does not correct spelling and searches only cold laser related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- Where ever possible, the detailed section about the resource will link to the sources.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic lasers. It does include some resources on weight loss and smoking cessation.
The results of the search are sorted based on 3 quality factors on a scale of 1 to 10 with 10 being the best score. Originally all the resources were given a 5-5-5 until they could be individually evaluated. These scores are purely opinion and are only used to simplify the rank of the results from more valuable to least valuable. This should not be considered a critique of any work. This system was created to help researchers (including ourselves) find the most usable resources for any cold laser therapy research. The resources are assigned values based on the following 3 factors:
- Efficacy: The resource (especially research papers) should show a significant improvement in the condition being treated. Resources that show better results are given a higher quality score.
- Detail: The source must give enough information that the results can be duplicated. If a resource lacks too many details that it cannot be recreated, it is given a lower detail score.
- Lack of Bias: Many resources are created to try and show that one device is superior to its competition. Many manufacturers have staff that crank out biased papers on a regular basis on the hope that this will make their product look superior. If the author of the resource is paid by a manufacturer of the resource appears to be biased towards one device and not one technology, the resource has much less value.
Over the past few years of working with research, we found that a majority of the published resources are lacking in one of these three ranking factors.
The original goal of this research tool was to tie published resources to the protocols in the laser-therapy.us library. This connection allows users to trace each protocol back to a list of resources so the protocol can be researched and improved.
General Comments
POWER
When many of the first research papers were published, the most power laser available for therapy were less than 100mW and many systems had to be pulsed to keep the laser from burning out too quickly. Today, system are available that will deliver up to 60,000mW of continuous output. Because of these power limitation, many early studies were limited to extremely low dosages by today’s standards. It takes a 50mW system 17 minutes to deliver 50 joules at the surface of the skin. If this was spread over a large area of damage or was treating a deeper problem, the actual dosages were much less than 1J/cm2. Today, we know that these dosages typically produce very little or no results.
WAVELENGTH
About 80% of the resources in this database are in the near infrared wavelength. There is also some interest in the red wavelength (600 to 660nm) . Other wavelengths like blue, purple, and green have very little scientific research behind them and have not gotten much traction in the core therapy market with the exception of some fringe consumer products.
Legal Disclaimer
This research tool is free to use but we make no claims about the accuracy of the information. It is an aggregation of existing published resources and it is up to the user to determine if the source of the resources has any value. The information provided through this web site should not be used for diagnosing or treating a health problem or disease. If you have or suspect you may have a health problem, you should consult your local health care provider.
Welcome to the BioPhotonica Education Center. There are over 5000 successful studies showing the efficacy of PBM, light therapy and sound therapy. This is a searchable collection of technical publications, books, videos and other resources about the best practices in the industry and about treating a wide variety of problems. All the resources include links to the original source (where available) so we are not making any claims about the use of our technology for treating "non-FDA cleared" applications, we are simply summarizing what the expert are saying about proper application of these technologies.
Enter a keyword above and click on one of the following links to see a set of publications about that subject. HINT: Shorter keywords work better.
Here are some of our favorite queries:
- Whole Body Systems
- Treating Post Covid with PBMT
- Info for beginners (See several graphic representation of the PBM chemical process)
- Best wavelength for light therapy (980nm vs. 810nm vs 660nm...)
- Pulsing Versus continuous wave
- Dosage: Searching for the best practices for dosage
- Great summary of positive double blind studies
Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing athletic performance
Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Laser acupuncture
- Traumatic Brain Injury (TBI)
- Soft Tissue Injury
- Fertility and Reproductive Health
Autoimmune Research
Contraindications
This tool uses a broad match query so:
- It does not correct spelling and searches only PBM related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic therapies.
Welcome to the Lighthouse Health Education Center. There are over 5000 successful studies showing the efficacy of PBM, light therapy and sound therapy. This is a searchable collection of technical publications, books, videos and other resources about the best practices in the industry and about treating a wide variety of problems. All the resources include links to the original source (where available) so we are not making any claims about the use of our technology for treating "non-FDA cleared" applications, we are simply summarizing what the expert are saying about proper application of these technologies.
Enter a keyword above and click on one of the following links to see a set of publications about that subject. HINT: Shorter keywords work better.
Here are some of our favorite queries:
- Whole Body Systems
- Treating Post Covid with PBMT
- Info for beginners (See several graphic representation of the PBM chemical process)
- Best wavelength for light therapy (980nm vs. 810nm vs 660nm...)
- Pulsing Versus continuous wave
- Dosage: Searching for the best practices for dosage
- Great summary of positive double blind studies
Testimonials
- Amazing results
- Video testimonials from patients
- Video testimonials from doctors
- Incredible results in animals
- Increasing athletic performance
Research Info for other Applications
- Stem cell production in laser therapy
- Cosmetic and skin therapy parameters
- Laser acupuncture
- Traumatic Brain Injury (TBI)
- Soft Tissue Injury
Autoimmune Research
Contraindications
This tool uses a broad match query so:
- It does not correct spelling and searches only cold laser related subjects so do not use LLLT, cold or laser in the search bar
- It works better with shorter search terms or even parts of search terms
- It searches all the available fields so you can enter a body part, author, condition or laser brand.
- This system is only for photobiomodulation or cold laser therapy research (including LLLT, laser acupuncture and high power laser therapy) only. It does NOT include photodynamic laser therapy (where the laser is used to react with a pharmaceutical), hot surgery lasers or cosmetic therapies.

