Physical activity is recommended and beneficial for both asymptomatic persons and individuals with chronic diseases [1, 2]. Aerobic endurance is considered a useful tool for the assessment of physical fitness and the detection of changes in aerobic fitness resulting from systematic training [3].
Regular aerobic exercise has various beneficial metabolic, vascular, and cardiorespiratory effects [4]. Additionally, it decreases body fat and increases muscle mass, muscle strength, and bone density [5]. Moreover, it improves self-esteem and physical and mental health and reduces the incidence of anxiety and depression [4, 6].
Various ergogenic agents, such as whey protein [7], caffeine [8], creatine [9], and neuromuscular electrical stimulation [10], are currently used to increase the benefits of aerobic training. Photobiomodulation therapy (PBMT) has emerged as an electrophysical intervention that could be associated with aerobic training to enhance beneficial effects of aerobic exercise, since several studies used PBMT to improve physical performance when associated with different kinds of exercise [11, 12, 13, 14].
Several studies have recently used PBMT to improve muscle performance during aerobic activities in healthy adults [15, 16, 17, 18] and postmenopausal women [19, 20]. However, to the best of our knowledge, the best moment to perform irradiation with PBMT in aerobic training has not been yet elucidated.
For instance, the current literature shows that the application of PBMT before progressive aerobic exercise has ergogenic effects and acutely increases the time until exhaustion, covered distance, and pulmonary ventilation and decreases the score of dyspnea during progressive cardiopulmonary test [15]. In addition, PBMT irradiation performed prior to aerobic exercises improves the exercise performance by decreasing the exercise-induced oxidative stress and muscle damage [18] and increasing the oxygen extraction by peripheral muscles [16]. When performed during aerobic training sessions, PBMT improves the quadriceps power and reduces the peripheral fatigue in postmenopausal women [19, 20]. Additionally, when applied after the sessions of endurance-training program, PBMT leads to a greater fatigue reduction than endurance training without PBMT irradiation [17].
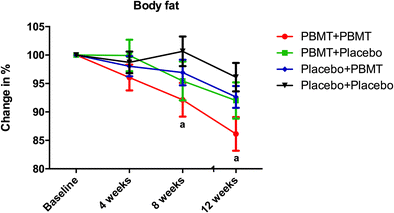
Therefore, the optimal moment to perform PBMT in aerobic training is still open to discussion. With this perspective in mind, we aimed to assess the effects of PBMT applied at different time points (before and/or after) of each training session and its potential effects on the outcomes of an endurance-training program (aerobic exercise).